/data/photo/2020/10/02/5f76818c5dc38.jpg)
[ad_1]
KOMPAS.com US President Donald Trump tested positive for coronavirus and is currently receiving treatment at Walter Reed Military Hospital.
Some foreign media reported that Trump had received the first dose of remdesivir.
In addition to receiving the drug, this New York-born man has also been injected with cocktails of Regeneron polyclonal antibodies, which contain famotidine, melatonin, aspirin, zinc and vitamin D.
Also read: Covid-19 positive, what kind of treatment is given to Trump?
So what is remdesivir?
Launching Kompas.com (10/5/2020, remdesivir has been approved by the US government in emergency use related to Covid-19.
Under an emergency use authorization granted by the US Food and Drug Administration (FDA) on May 1, 2020, hospitals can administer remdesivir intravenously to patients using ventilators or in need of supplemental oxygen assistance. .
Remdesivir is the first drug approved to treat Covid-19 disease.
Also Read: Over 1 Million Positive Cases, US Allows Remdesivir To Be Used As Covid-19 Drug
The drug produced by Gilead Sciences is claimed to accelerate the recovery time of patients infected with the corona virus.
In addition, the drug remdesivir was able to shorten recovery time in 1,063 patients with an average of about four days of hospitalization.
This drug was originally tested as an antiviral against Ebola and hepatitis C.
Also read: Look at how Ebola helps Africa deal with the coronavirus …
How redemsivir works
 The illustration of remdesivir that was originally developed for the antiviral drug Ebola, again shows positive clinical trial results in its use to treat new coronavirus infections in Covid-19 patients.
The illustration of remdesivir that was originally developed for the antiviral drug Ebola, again shows positive clinical trial results in its use to treat new coronavirus infections in Covid-19 patients.Redemsivir is one of the drugs that enter standard of care World Health Organization (WHO).
This drug should not be given carelessly to all Covid-19 patients.
Redemsivir is only indicated for laboratory-confirmed Covid-19 patients, especially adults or adolescents 12 years of age or older with a minimum body weight of 40 kilograms.
Also read: When the US started distributing Remdesivir to Covid-19 patients in 6 states …
This drug will interfere with the replication of the new virus by inserting it into new viral genes.
It is said that redemsivir can inhibit viral replication so that it no longer occurs and the patient’s immune system can control the virus.
Although it is claimed that it may inhibit viral replication and shorten recovery time for Covid-19 patients, the use of remdesivir increases side effects.
The side effects of using this drug are believed to affect the liver, liver, and even kidneys.
Experts also caution that the drug, which was originally developed to treat Ebola and manufactured by the pharmaceutical company Gilead, should not be viewed as the only alternative to the coronavirus drug.
Also read: After Remdesivir, Japan approves that dexamethasone is a Covid-19 drug
Remdesivir in Indonesia
 Illustration of Remdesivir for Covid-19 patients.
Illustration of Remdesivir for Covid-19 patients.Redemsivir is scheduled to be distributed in Indonesia in the near future.
PT Kalbe Farmas Tbk is the distributor of the drug redemsivir which is produced by India’s leading pharmaceutical company Hetero.
The price of an antiviral drug with the Covifor trademark is priced at IDR 3 million per vial or per dose.
A vial is a container for liquid, powder, or pharmaceutical tablets. Modern vials are generally made of glass or plastic.
The drug covifor remdesivir will only be sold and marketed in hospitals.
Also read: Covid-19, the treatment of Donald Trump and the use of Remdesivir …
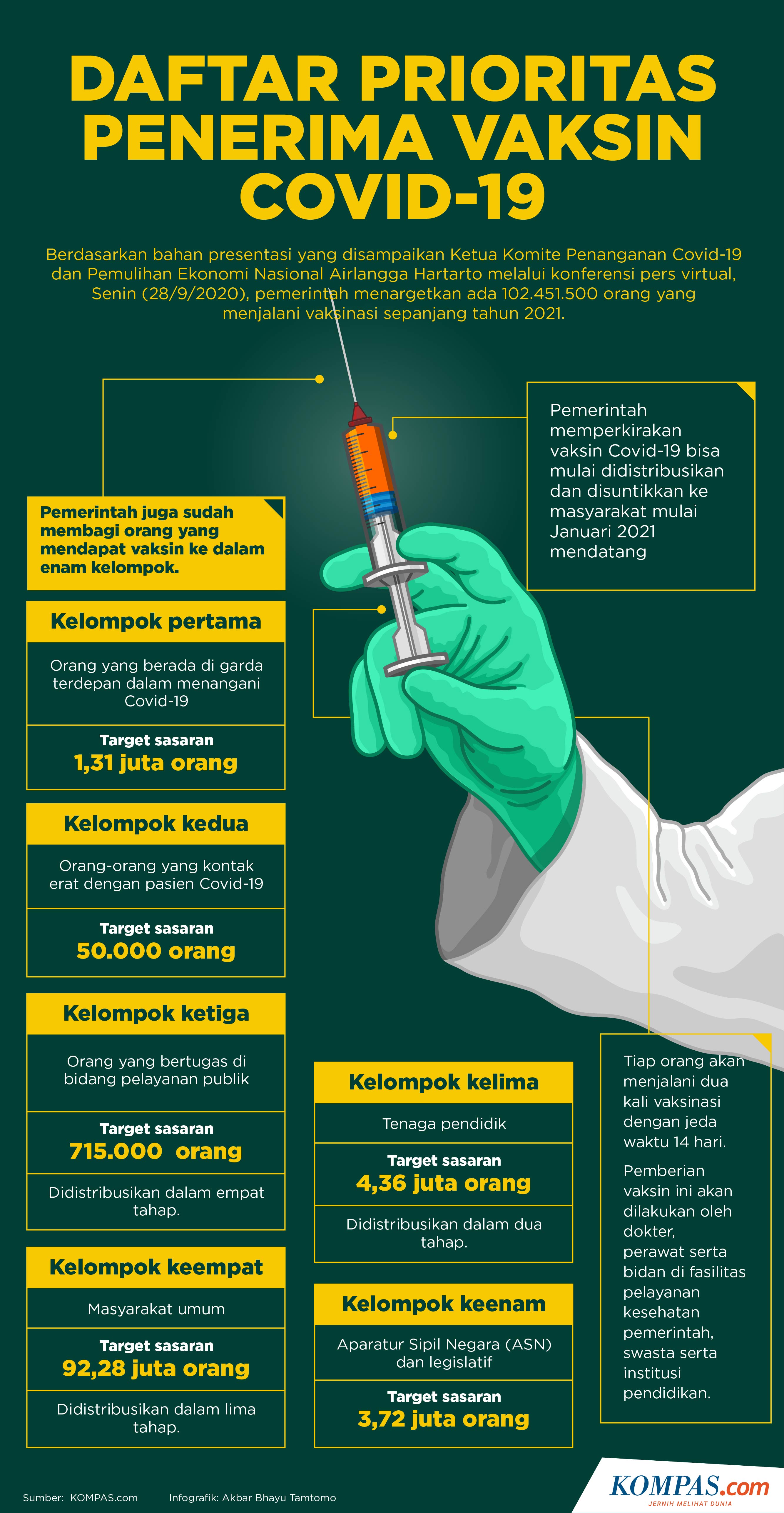
Infographic: Priority List of Covid-19 Vaccine Recipients