
“The efficacy and safety of this vaccine confirm that it will be highly effective against Covid-19 and will have an immediate impact on this public health emergency,” Pascal Soriot, Astra’s CEO, said in a statement.
The vaccine developed by the University of Oxford was 90% effective in preventing Covid-19 when administered as a half dose followed by a full dose at least a month apart, according to data from late-stage trials in Gran Britain and Brazil.
Another dosage regimen showed an efficacy of 62% when administered as two full doses separated by at least one month, and the combined analysis of both dosage regimens resulted in a mean efficacy of 70%.
Incredibly exciting news that the Oxford vaccine has been shown to be so effective in trials. There are still more security checks … https://t.co/yThliyInIA
– Boris Johnson (@BorisJohnson) 1606117502000
The encouraging results are expected to come as a shot in the arm for India, as it has the inexpensive Oxford vaccine to inoculate most of its population.
The Oxford candidate is being manufactured locally by the Serum Institute of India, which aims to deliver the first of 100 million doses to healthcare workers and the elderly by January next year.
Reacting to the announcement, Serum CEO Adar Poonawalla said he is delighted to learn that Covishield, the local name for the vaccine, will soon be widely available.
“I am delighted to hear that Covishield, a low-cost, logistically manageable and soon to be widely available # COVID19 vaccine, will offer protection up to 90% in one type of dosage regimen and 62% in the other dosage regimen. More details on this will be provided tonight, “Poonawalla said on Twitter.

The vaccine has a significant advantage over candidates like Moderna and Pfizer, which were also found to be more than 90% effective, in terms of cost and storage.
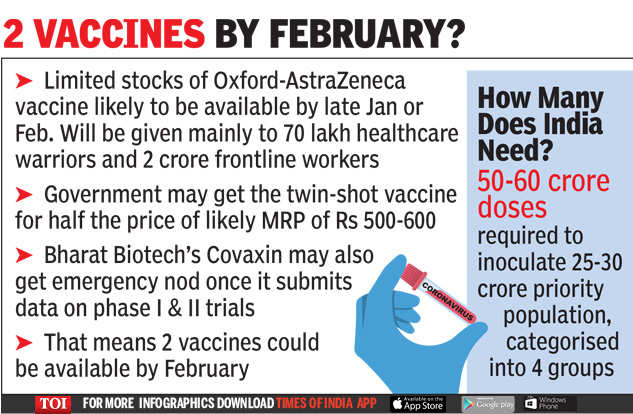
India is likely to receive the first batch of anti-Covid injections from January-February next year, allowing some front-line workers, such as doctors, nurses and municipal staff, to receive the vaccine. This will be possible as India plans to grant Serum Institute of India (SII) emergency use approval for the Oxford-AstraZeneca vaccine candidate shortly after gaining similar approval in the UK.
The government, which will be making bulk purchases, has also negotiated a better price, almost half the likely MRP of Rs 500-600 for the two-shot vaccine, an official source said.
In addition, the puncture can be kept at refrigerator temperatures, while those from Pfizer and Moderna, based on novel messenger RNA technology, require freezing for longer-term storage and transport.
The Oxford University vaccine is based on a harmless, weakened version of a common cold virus, or adenovirus, that causes infections in chimpanzees. The vector (the carrier) is derived from adenovirus (ChAdOx1) extracted from chimpanzees. It is genetically engineered so that it does not reproduce in humans.
.