[ad_1]
Here is all you need to know about sero-survey–
What is sero-survey?
A sero-survey involves testing of blood serum of a group of individuals and this will be used to monitor trends in prevalence of the novel coronavirus, or SARS-COV-2, infection at the district level. The surveillance will be conducted by the Indian Council of Medical Research and the National Center for Disease Control in collaboration with key stakeholders and state health departments.
The facility-oriented surveillance is an expansion of the testing of flu and serious respiratory cases in hospitals being carried out by the government.
Why sero-testing?
A more focused population-based sero-survey of high and low-risk groups in select districts will be in addition to routine testing.
The move will not only help the government and its agencies monitor Covid-19 trends but also check for community transmission in any part of the country.
The health ministry has so far maintained that there is no evidence yet of community transmission in the country. There are large outbreaks in some clusters but the sharp exponential rise in cases as in community transmission has not happened.
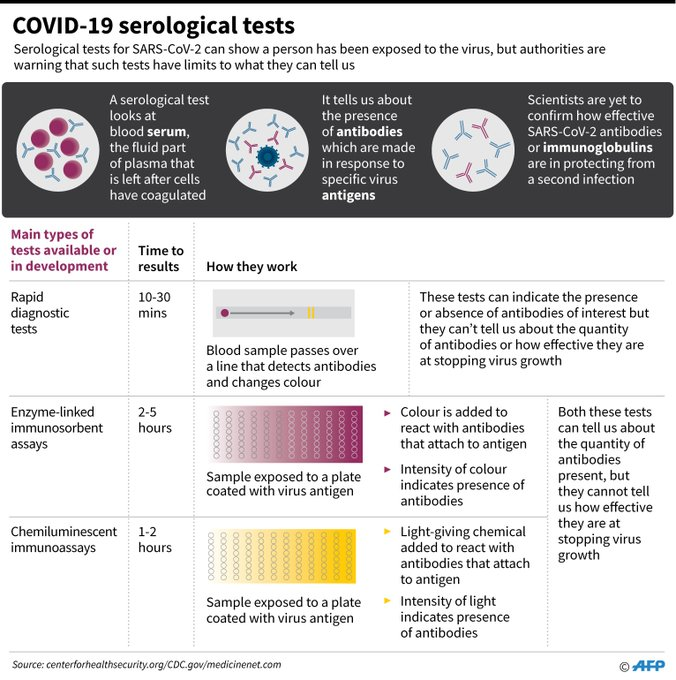
How will it be conducted?
The enhanced exercise will see 10 health facilities – six public and four private – from each district being tested.
For the sero-survey, population groups will consist of low- as well as high-risk populations. The low-risk group will include outpatient attendees (non-ILI patients) and pregnant women, while healthcare workers will be surveyed among the high-risk population.
The survey will include a total of 200 samples per week and 800 samples per month. This will include at least 100 samples per week and 400 per month from the selected districts from the high-risk population. For the low-risk population, 50 samples per week and 200 per month are to be collected from outpatient attendees (non-ILI patients).
Using a combination of RT-PCR and Elisa kits
The agencies will use a combination of RT-PCR and Elisa antibody kits for these surveys. Throat and nasal swabs will be collected for RT-PCR tests and samples tested in a one-time pool of 25. However, the results of this sample pooling are only for surveillance purposes and not for diagnosis of individual patients.
In addition to RT-PCR tests, blood samples will be collected for detecting IgG antibodies for Elisa testing. In subsequent rounds, IgG Elisa-based testing of serum samples will replace RT-PCR based testing for surveillance purposes.
Elisa testing kit to replace rapid antibody test kits from China
The Elisa testing kit has been developed by National Institute of Virology in Pune along with Zydus Cadila. The kit is expected to play a critical role in surveillance of a proportion of the population exposed to the infection, after rapid antibody test kits from China failed the ICMR quality tests. Health ministry joint secretary Lav Agarwal said the Elisa kits have specificity of 97% and sensitivity of 92%.
.
[ad_2]