
Updated: October 7, 2020 8:28:07 am
 A medical worker wearing protective gear disinfects his colleague after accompanying an ambulance patient to a hospital for Covid-19 treatment in St. Petersburg, Russia, on Thursday, Oct. 1, 2020 (AP Photo: Dmitri Lovetsky) .
A medical worker wearing protective gear disinfects his colleague after accompanying an ambulance patient to a hospital for Covid-19 treatment in St. Petersburg, Russia, on Thursday, Oct. 1, 2020 (AP Photo: Dmitri Lovetsky) .
Coronavirus Vaccine Tracker: The United States’ drug regulator, the Food and Drug Administration (FDA), has asked developers of the coronavirus vaccine to monitor the health of trial participants for at least two months before seeking approval.
The new stricter guidelines issued by the FDA on Tuesday almost rule out every chance that a vaccine will be available, or approved, before the U.S. presidential election on November 3, something that President Donald Trump has repeatedly said it was possible.
The guidelines were finalized and submitted for the president’s approval about two weeks ago, but the White House had been sitting on them all this time, according to a report in The New York Times. The approval finally came on Tuesday.
Four main candidates for the coronavirus vaccine are currently in phase 3 clinical trials, the last stage of testing, in the United States. Pfizer it has said that it hopes to obtain reliable data on the efficacy of its vaccine in October itself. You have said that if the effectiveness data were satisfactory, you would immediately request emergency use approval for your vaccine. Pfizer’s schedule had sparked speculation that a vaccine could be approved before the November 3 date.
However, the new FDA guidance could delay such ambitious deadlines. The new guidelines also ask developers to submit additional data on their trial participants, including those who have been given a dummy instead of the actual vaccine.
The trials of AstraZeneca The vaccine, suspended a month ago after one of the trial participants in the UK developed a serious illness, is yet to be resumed in the United States. The US authorities have ordered an investigation into the entire episode, despite the fact that trials have been restarted in other countries, the United Kingdom, Brazil, South Africa and India.
The other two companies, whose vaccines are in phase 3 trials, Modern Y Johnson and Johnson, have said that they expect their vaccine to be ready early next year.
📣 Express explained is now in Telegram. Click here to join our channel (@ieexplained) and stay updated with the latest
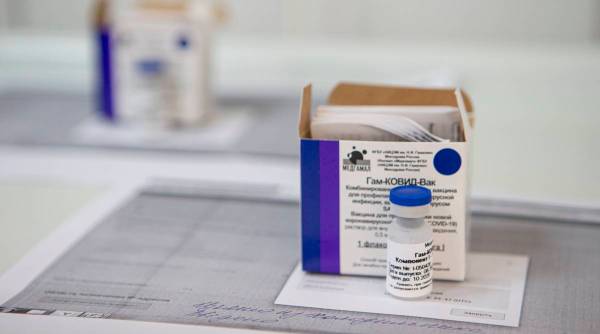 A view of a vial of Russia’s experimental Sputnik V coronavirus vaccine in Moscow, Russia. (AP Photo)
A view of a vial of Russia’s experimental Sputnik V coronavirus vaccine in Moscow, Russia. (AP Photo)
Russia speeds up second coronavirus vaccine
Russia has been developing another coronavirus vaccine, the second, and is accelerating its development to secure approval by the middle of this month, a report in The Wall Street Journal He said.
Russia’s first vaccine, approved by the country’s regulator on August 12, had been widely criticized because it had not undergone phase 3 clinical trials, something that is just starting now. It was later clarified that the approval was only conditional and for emergency use, and that it depended on phase 3 trials being carried out later.
That vaccine is already being administered in Russia and is expected to be used by other countries as well, including India. Dr Reddy’s Laboratories, based in Hyderabad, has entered into an agreement distribute the vaccine in India. But before it can be used in the Indian population, the Russian vaccine, as is the case with any vaccine developed outside the country, would have to undergo advanced stage clinical trials in India. Those trials are expected to begin next month.
The Wall Street Journal He said the second vaccine was being developed by a former Soviet biological weapons research laboratory called the Vector State Virology and Biotechnology Center. He quoted the Russian Health Minister as saying that he expected the vaccine to gain approval in mid-October.
Search for the coronavirus vaccine: the story so far
- 193 candidate vaccines in clinical or preclinical trials
- 42 of them in clinical trials
- Ten in final stages, phase III human trials
- At least eight potential vaccines are under development in India. Two of them have entered phase II trials after completing phase I.
The most commented:
* AstraZeneca / University of Oxford
* Modern
* Pfizer / BioNTech
* Johnson and Johnson
* Sanofi / GlaxoSmithKline
* Novavax
* Russian vaccine, developed by the Gamaleya Institute in Moscow
* Three Chinese vaccines that have been approved for use in China without the completion of phase 3 trials. One of them has received authorization for emergency use in the UAE.
(As of October 6; source: WHO Coronavirus Vaccine Overview of October 2, 2020)
📣 The Indian Express is now on Telegram. Click here to join our channel (@indianexpress) and stay up to date with the latest headlines
For the latest news explained, download the Indian Express app.
© The Indian Express (P) Ltd
.