
Updated: September 7, 2020 8:41:47 am
 A nurse holds China’s Sinovac vaccine, a possible vaccine for the coronavirus disease (COVID-19), at the Sao Lucas Hospital in Brazil (Reuters / Archive)
A nurse holds China’s Sinovac vaccine, a possible vaccine for the coronavirus disease (COVID-19), at the Sao Lucas Hospital in Brazil (Reuters / Archive)
Coronavirus Vaccine Tracker (COVID-19): A coronavirus vaccine being developed by a Chinese company was used for the first time on its own employees and their families. This vaccine, developed by Sinovac Biotech, is one of three that China has authorized for “limited” or emergency use, without phase 3 clinical trials.
A Reuters report quoted the company’s chief executive as saying that about 90 percent of employees and their families had received doses of the vaccine. The CEO said the vaccine was “offered” to approximately 2,000 to 3,000 employees and their families, “on a voluntary basis.” It was not clear exactly how many people were injected with the vaccine.
📣 Express explained is now in Telegram. Click here to join our channel (@ieexplained) and stay up to date with the latest
The Sinovac vaccine, called CoronVac, was approved for emergency use in July, but only now started phase 3 trials that take place in Brazil and Indonesia.
Two other candidate vaccines, one of which is being developed by Sinopharm, and the other for CanSino Biologics in collaboration with the Academy of Military Medical Sciences, the Chinese authorities have also been approved. There is no information on the number of people who received these other two vaccines. These two candidates have also not gone through phase three clinical trials, which are just beginning now.
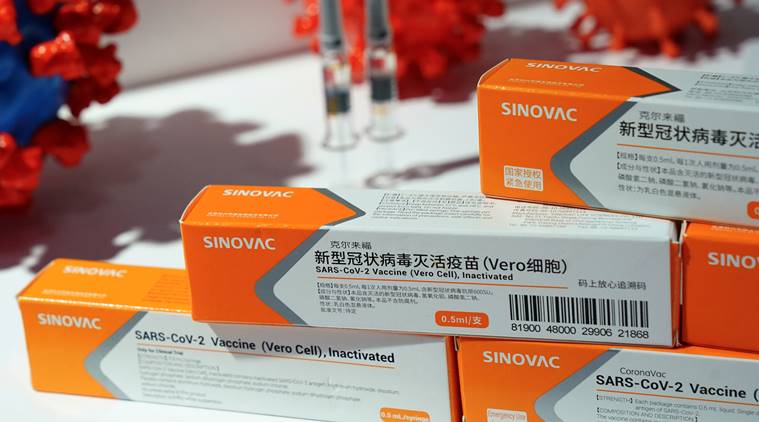 A booth displaying a coronavirus vaccine candidate from Sinovac Biotech Ltd is seen at the 2020 China International Trade in Services Fair (CIFTIS), following the COVID-19 outbreak, in Beijing (Reuters)
A booth displaying a coronavirus vaccine candidate from Sinovac Biotech Ltd is seen at the 2020 China International Trade in Services Fair (CIFTIS), following the COVID-19 outbreak, in Beijing (Reuters)
Of the 34 vaccines currently in clinical trials, eight are being developed by Chinese companies or institutions.
Search for the coronavirus vaccine: the story so far
- More than 175 vaccine candidates in preclinical or clinical trials
- 34 of them in clinical trials
- Eight in final stages, phase III human trials
- At least eight potential vaccines are under development in India. Two of them have entered phase II trials after completing phase I.
(As of Sept. 3; source: WHO Coronavirus Vaccine Overview Sept. 3, 2020)
Read also | Extremely unlikely but not impossible shot before US elections, says WH adviser
📣 The Indian Express is now on Telegram. Click here to join our channel (@indianexpress) and stay up to date with the latest headlines
For the latest news explained, download the Indian Express app.
© IE Online Media Services Pvt Ltd
.