November 21, 2020 11:51:38 am
 Pfizer and state and federal government officials have been preparing to help distribute the vaccine within days of an emergency clearance from the FDA. (Bloomberg)
Pfizer and state and federal government officials have been preparing to help distribute the vaccine within days of an emergency clearance from the FDA. (Bloomberg)
Pfizer Inc. and BioNTech SE applied for emergency clearance for their Covid vaccine on Friday, and it could take at least three weeks for a decision from the U.S. Food and Drug Administration, as agency staff and external consultants investigate the trial data.
With 95% efficacy and no major safety concerns, your vaccine may be the first to be approved for use, but it must first undergo a thorough review. The presentation could allow its use from mid to late December, the companies said in a statement.
A key step along the way is a meeting of outside FDA advisers, all experts in infectious diseases and vaccines. The FDA will convene the advisory group on Dec. 10 to discuss the Pfizer and BioNTech vaccine, according to an agency statement Friday. The FDA will spend the few weeks between the emergency clearance request and the meeting to classify the trial data.
“I do not find that time frame unreasonable in light of the amount of data the agency needs to analyze and to ensure full participation of all stakeholders on the advisory committee,” said John Taylor, who served as an advisor to the Chief of the FDA during the Obama administration and now works as a consultant at Greenleaf Health. “It ensures that the agency can run a well-run and well-attended advisory committee to ensure maximum transparency, which I think will be a major factor in the willingness of patients to receive the vaccine.”
FDA Commissioner Stephen Hahn also said earlier this week that the agency will release documents related to its review of any vaccine that has been granted emergency clearance. The goal is “to contribute to public confidence in the agency’s rigorous review of scientific data and the appropriate use of licensed products.”
Pfizer gained 1.4% in New York, while BioNTech’s US depository receipts increased 9.6%.
Key moments
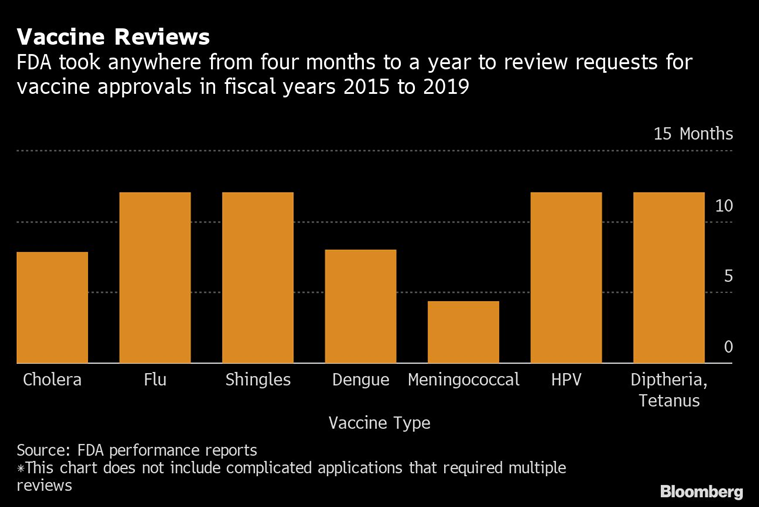
The emergency authorization process allows drugs and vaccines directed against Covid-19 to reach Americans much faster than through standard approval channels. The FDA review process typically takes between six and ten months, depending on the candidate’s priority status. Some treatments, such as certain anticancer drugs, are cleared in just a couple of months.
Pfizer and state and federal government officials have been preparing to help distribute the vaccine within days of an emergency clearance from the FDA. The company’s vaccine will require special freezers to transport and store.
There are two key moments to pay attention to during an FDA advisory panel meeting. The first occurs two days before the assessors meet, when the FDA typically publishes its staff report on the clinical trial data. This will provide an idea of whether the agency is leaning toward licensing the vaccine. The second is at the end of the meeting, when a non-binding vote will be taken on whether the FDA should authorize the public use.
FDA staff will review Pfizer’s raw data, which the public does not have access to, rather than relying on the company’s own results to determine the safety and efficacy of the vaccine. The agency and Pfizer will each summarize their findings for the advisory panel.
Moderna Inc. has also released positive interim results from an end-stage trial and said it is close to requesting emergency authorization for its Covid-19 vaccine. The company has said that it expects the final results of the study in about a week or two.
In the event that the FDA grants emergency clearance shortly after the meeting, there are some steps the agency would normally take for a vaccine, but in this case they can be skipped. Under the regular vaccine approval process, the FDA would collect quality test results from a company, as well as samples that the agency could test to make sure they meet US standards. publish to the public.
Since the first Covid-19 vaccines will not go through the regular approval process, but will instead be authorized in an emergency, the FDA may forego collecting the data and samples. The agency will make the decision on a case-by-case basis, a spokeswoman said.
The FDA has also said that emergency use regulations do not require them to conduct inspections of manufacturing plants prior to a vaccine authorization, as they normally would with a regular approval. Inspections are used to verify that drug or vaccine manufacturers can produce quality products.
Founded in 2010, Moderna has yet to obtain FDA clearance for any product. An FDA database does not indicate that Moderna has been inspected by the agency.
The FDA is not the only US agency to consider vaccines. Another group of advisers from the Centers for Disease Control and Prevention should also meet about the vaccine and make recommendations on who should be the first to get it. The timing of this meeting is unclear.
Arkansas Health Secretary Jose Romero, who chairs the CDC’s Advisory Committee on Immunization Practices, said in an email through a spokeswoman Wednesday that the panel is maintaining a plan to meet shortly after the FDA grants the emergency authorization for a Covid-19 vaccine. Health and Human Services Secretary Alex Azar also said Wednesday that there is an effort to match the CDC advisory meeting with that of FDA advisers. Azar’s office did not respond to requests for comment.
Health workers first
Health workers are expected to be the first to receive a vaccine. Between Pfizer and Moderna, Azar said, the US should have access to about 40 million doses by the end of December, enough for 20 million people to receive the two-dose regimen.
The CDC estimates that there are between 17 and 20 million healthcare workers in the US, according to a presentation that Kathleen Dooling, an epidemiologist with the agency, gave to ACIP in August.
As doses of the vaccine begin to be distributed, government involvement will not end there. The FDA has said it expects manufacturers to apply for regular approval as soon as possible. This will require pharmaceutical companies to continue their studies, even after applying for an emergency authorization.
The agency is allowing them to obtain an initial authorization based on two months of security data; Six months of data is expected in an application for full approval, Doran Fink, deputy director of the FDA’s clinical division for vaccine applications and related products, told agency advisers at a meeting in October.
Once Americans start taking a vaccine, it is expected that pharmaceutical companies will find it difficult to continue conducting trials that include a group of volunteers who are given a placebo instead of the vaccine. The FDA is currently considering alternatives, Fink said.
.