Updated: October 3, 2020 10:31:51 pm
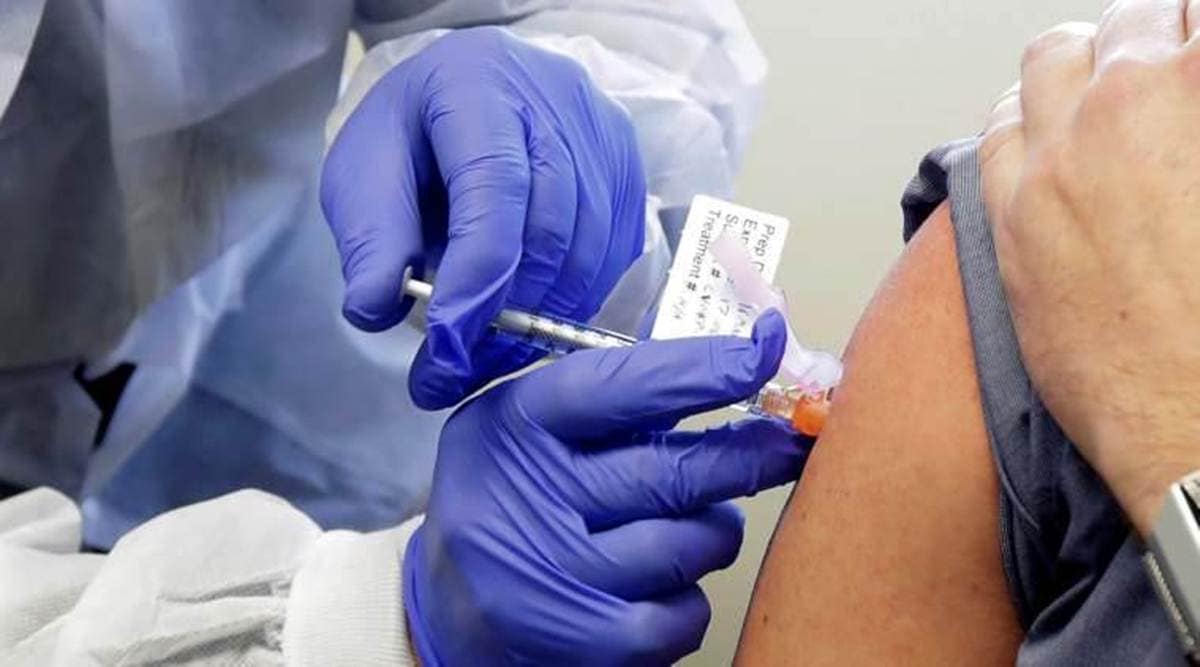
 The candidate vaccine tested by scientists at the University of Oxford in collaboration with the pharmaceutical giant AstraZeneca is the most advanced in the testing process and, according to a report by “The Times”
The candidate vaccine tested by scientists at the University of Oxford in collaboration with the pharmaceutical giant AstraZeneca is the most advanced in the testing process and, according to a report by “The Times”
There is growing hope that health regulators will give a green light for a coronavirus vaccine later this year to roll out into a vaccination program in six months or even less, according to a report by UK media. .
The candidate vaccine being tested by scientists at the University of Oxford in collaboration with the pharmaceutical giant AstraZeneca is the most advanced in the testing process and, according to a report by ‘The Times’, could receive the required authorizations by Christmas in December .
The newspaper quoted UK government sources involved in vaccine manufacturing and distribution as saying that a full adult vaccine rollout program could take six months or less after approval.
“We are looking at about six months and it is likely to be much shorter than that,” said a government source.
Read | Quixplained: How Much Will Covid-19 Vaccines Cost in India?
Under a protocol developed by the UK Joint Committee on Vaccination and Immunization, any approved vaccine will be given to everyone over the age of 65, followed by the youngest adults at highest risk, which could include those from ethnic minorities and those with serious health problems. about your increased risk of the deadly virus. People over 50 will be next in line, with the youngest adults at the end of the line.
The UK government has ordered 100 million doses of the Oxford vaccine once it is ready for deployment and the doses are being manufactured before they have been shown to be successful in saving time once it passes all regulatory stages.
Read | First dose of Oxford Covid-19 vaccine administered to 3 people at PGIMER
According to the newspaper report, the trial scientists hope to get results before the end of this year and at least show that it prevents 50 percent of infections, the threshold of success.
If approved by regulators, the UK’s National Health Service (NHS) is said to be in a position to start mass vaccination almost immediately.
However, there are others within the UK government who are more cautious about deadlines, as vaccinating all adults is a big challenge. A Royal Society report this week, co-authored by an Indian-born scientist, warned of the enormous and arduous task ahead of us to produce and distribute a vaccine.
“Even when the vaccine is available, it does not mean that within a month everyone will be vaccinated. We are talking about six to nine months to a year after a vaccine is approved, ”said Professor Nilay Shah, director of chemical engineering at Imperial College London.
Read also | Quixplained: When will a coronavirus vaccine be ready?
The report called for criteria for vaccine prioritization to be “defined and made explicit” and then “public dialogue and engagement to manage expectations and understanding of vaccine efficacy, safety, side effects, availability and access. “.
The government’s Health Department tried to downplay the findings, emphasizing that its planning process will ensure speedy implementation.
“This study does not reflect the enormous amount of planning and preparation that has been done throughout the government to rapidly implement a safe and effective COVID-19 vaccine,” a department spokesman said.
“We trust that we have an adequate supply or transport [personal protective equipment] and logistics expertise to implement a Covid-19 vaccine across the country as quickly as possible, ”the spokesperson said.
📣 The Indian Express is now on Telegram. Click here to join our channel (@indianexpress) and stay up to date with the latest headlines
For the latest world news, download the Indian Express app.
.