
Updated: September 7, 2020 9:27:45 pm
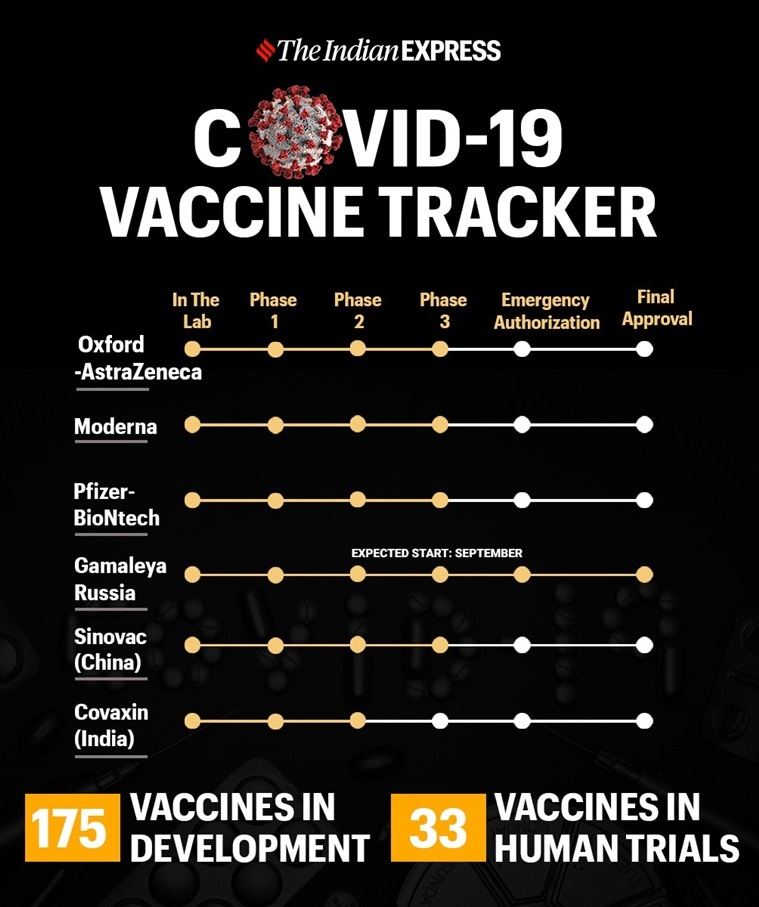
 According to the World Health Organization, about 175 vaccine candidates are in preclinical or clinical trials today.
According to the World Health Organization, about 175 vaccine candidates are in preclinical or clinical trials today.
While Russia and China may have stolen a march in the race to develop a Covid-19 vaccine, grant regulatory approval to vaccine candidates Even before phase III human trials, all eyes are on Pfizer’s other leading vaccine candidates, Oxford University-AstraZeneca and Moderna. All three are currently in the late testing phase and it has become clear that a vaccine would be available this year.
According to the World Health Organization, about 175 vaccine candidates are currently in pre-clinical or clinical trials. About 33 of them are in the clinical trial phase. Eight contenders are in the final stages, phase III of human trials. At least eight candidate vaccines are under development in India, of which two are in phase II testing phase.
Covid-19 Vaccine from University of Oxford-AstraZeneca
The ChAdOx1 nCoV-19 vaccine, considered a pioneer in the global race to provide an effective vaccine to combat Covid-19, is now in phase III clinical trials in Great Britain, Brazil, South Africa and India. The vaccine is made from a weakened version of a common cold virus, called adenovirus, that infects chimpanzees.
Latest updates:


Australian Prime Minister Scott Morrison said the country would receive the first batches of the potential Covid-19 vaccine from Oxford in January. “Australia will receive 3.8 million doses of the vaccine in January and February 2021,” he said.
Pfizer-BioNTech Covid-19 Vaccine
The BNT162b2 vaccine being developed by Pfizer, in conjunction with German biotech company BioNTech, is also undergoing phase 3 trials. The vaccine uses messenger RNA for the immune system to recognize the coronavirus. Pfizer recently said that the first data from its phase 3 trials will be available in October.
Latest updates:



Modern Covid-19 Vaccine
The mRNA-1273 vaccine developed by the American biotech company Moderna is undergoing phase III trials in which scientists will evaluate a 100 µg dose of the candidate vaccine in about 30,000 participants in the US Moderna Inc plans to put a price of $ 50 to $ 60 per course for your coronavirus vaccine.
Latest updates:



Gamaleya Institute, Russia Covid-19 Vaccine
The Sputnik V vaccine developed by the Gamaleya Institute in Moscow is the first in the world to be licensed for general use. It was approved by the Russian government on August 11, without phase 3 trials, drawing global criticism. The vaccine uses two adenoviruses to inject the genetic material of the new coronavirus into humans in order to trigger an immune response.
Latest updates:




Covaxin from Bharat Biotech
India’s first indigenous Covid-19 vaccine, Covaxin from Bharat Biotech, will begin phase II human trials. Covaxin, an “inactivated” vaccine, works by injecting doses of the virus that have been destroyed so that the body produces antibodies against it without the virus posing a threat.
Latest updates:


The different phases of the vaccine test:
Preclinical tests: At this stage, scientists test the vaccine in the laboratory using cells or animals.
Phase I trials: This is the first step in which the experimental vaccine is administered to humans and the safety, dosage, and possible side effects are examined. This stage generally takes about two months and involves a small number of participants, usually 20 to 100 healthy volunteers.
Phase II trials: At this stage, several hundred people sign up for the test and are divided into age groups, such as children and the elderly. This stage studies the immunogenicity of the candidate vaccine, the proposed doses, the immunization schedule and the method of administration.
Phase III trials: At this stage, the candidate vaccine is tested in hundreds or thousands of volunteers to determine its ability to prevent infections in humans in real-life situations (outside of laboratory conditions). During a pandemic, a vaccine can receive emergency use authorization before a formal green sign.
Approval: After Phase III testing, the developer submits a license request to the regulatory authority of their respective country. The regulator then inspects the factory where the vaccine will be made and approves its labeling.

For the latest news on coronavirus outbreaks, download the Indian Express app.
© IE Online Media Services Pvt Ltd
.