
Updated: September 11, 2020 7:29:25 am
 Covid-19 testing in New Delhi. IBS Sponsors Mid- and Late-Stage Human Clinical Trials for Candidate Vaccine in India
Covid-19 testing in New Delhi. IBS Sponsors Mid- and Late-Stage Human Clinical Trials for Candidate Vaccine in India
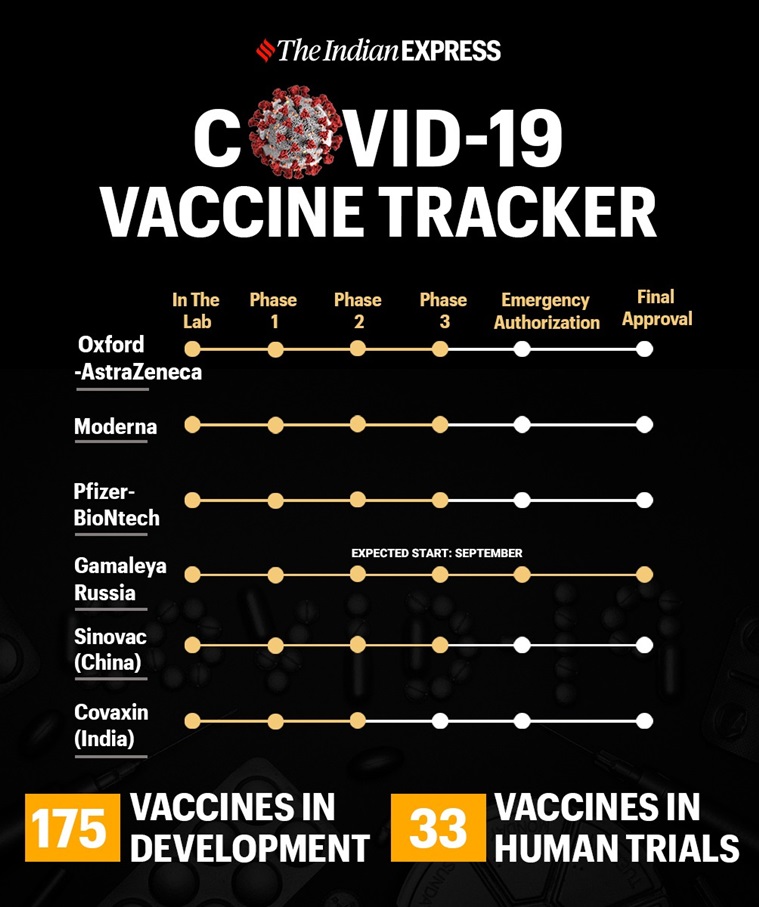
Serum Institute of India has decided to stop the ongoing clinical trials in India of the Covid-19 candidate developed by the University of Oxford until AstraZeneca restarts its own global tests of the vaccine. The development comes a day after the Pune-based firm announced that the Swedish-British pharmaceutical giant’s decision to pause ongoing trials while reviewing a potential safety issue would not affect ongoing trials in India.
The development comes a day after the The Comptroller General of Drugs of India (DCGI) issued a notice of show of cause to the Pune-based Serum Institute of India for failing to share information about the AstraZeneca vaccine pause. On Tuesday night, AstraZeneca had announced that it would temporarily halt global trials after a participant developed a “potentially unexplained illness.”
“We are reviewing the situation and stopping the trials in India until AstraZeneca restarts them. We are following the instructions of DCGI (Comptroller General of Drugs of India) and we will not be able to comment further on them, ”said Serum Institute.
IBS is sponsoring mid- and late-stage human clinical trials for the vaccine candidate in India. The candidate, named Covishield in India, was administered to a first group of volunteers on August 26.
AstraZeneca said it had “voluntarily paused vaccination” in its ongoing global trials to “allow for review of safety data by an independent committee” after the event triggered its standard review process.
Explained | What the AstraZeneca Vaccine Trial Pause Means for Vaccines
While the company did not specify which country had reported the adverse event, the issue is believed to have been noted in phase 2/3 clinical trials underway in the UK. One participant had reportedly developed a severe spinal inflammatory syndrome called transverse myelitis.
One of the conditions for SII to conduct the trials in India had been that the clinical data generated in India would be considered alongside the data from the trials conducted by Oxford-AstraZeneca, according to the advisory issued by DCGI Dr. VG Somani on Wednesday.
The regulator also noted that the SII had not submitted a “causality analysis” of the serious adverse event “in light of safety concerns.” Dr. Somani then asked the SII for an immediate response as to why the regulator’s permission to conduct the tests should not be suspended until patient safety is established. The chief had also threatened to “take measures deemed appropriate” in case the SII does not present an immediate response.
Prior to this show of cause notice, the SII spokesperson had said earlier Wednesday that, as far as the Indian trials are concerned, “it continues and we have not faced any problems.”
AstraZeneca and the University of Oxford contracted with the Pune company, the world’s largest vaccine manufacturer, to manufacture the vaccine for low- and middle-income countries.
Phase 2/3 trials of the candidate, named AZD1222 globally, have been conducted in the UK since the end of May. On September 3, AstraZeneca announced that the centers were recruiting up to 30,000 participants for phase 3 trials in the United States. Late-stage testing also continues in Brazil and South Africa, and the company has planned early to mid-stage testing in Japan and Russia.
Recruitment of participants in the Indian trials has already been on hold for the past week, as SII prepares to send the data collected from the first 100 participants given the vaccine for safety reviews, The Indian Express.
📣 The Indian Express is now on Telegram. Click here to join our channel (@indianexpress) and stay up to date with the latest headlines
For the latest news about India, download the Indian Express app.
© The Indian Express (P) Ltd
.