[ad_1]
No high-quality data demonstrating that taking antimalarial drugs hydroxychloroquine and chloroquine to be able to help COVID-19-19 patients, the authors of a review of existing evidence have warned. As such, they said the drugs should only be used to treat patients who participate in carefully designed clinical trials.
To conduct the review, lead author Dr. Mark Poznansky, director of the vaccine and Immunotherapy Massachusetts General Hospital Center, and colleagues observed anecdotal studies and reports on drug use in COVID-19-19 and other conditions. They released their findings in PHASEB daily, published by the Federation of American Societies for Experimental Biology.
“There is still no high-quality clinical data showing clear benefit from these agents” for COVID-19, the authors wrote.
Among their conclusions was that the drugs “have the potential to cause harm,” including serious heart problems when combined with other drugs. One preNon-peer-reviewed print study cited by researchers included more than 950,000 patients who took hydroxychloroquine. When combined with the antibiotic azithromycin, found that patients had a 15 to 20 percent higher risk of developing chest pain or heart failure in the first month of treatment, with the risk of dying from a doubled heart problem.
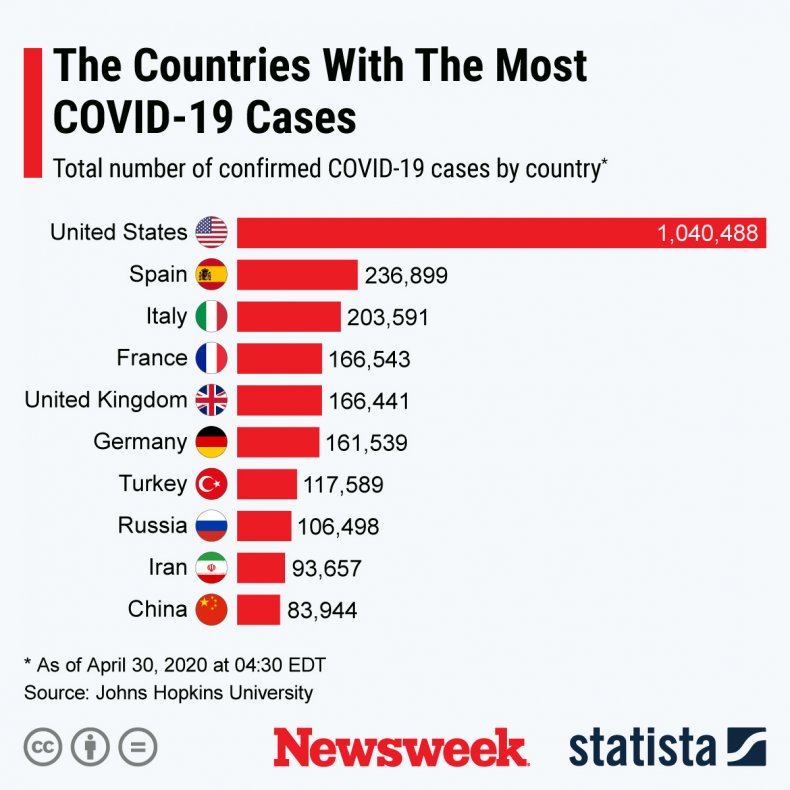
Currently there are no specific medications to treat COVID-19-19, who has sickened more than 3 million people worldwide, according to johns hopkins university. 228,057 people have died of the disease, and almost 1 million have survived. In the five months since COVID-19-19 the pandemic began, the United States has become the country with the best-known cases, such as graph below by Statistician shows.
Statista
In March the The Food and Drug Administration (FDA) issued the first Emergency Use Authorization regarding COVID-19-19, indicating that the two antimalarial drugs could be prescribed to adolescents and adults with the disease. Meanwhile, President Donald Trump has repeatedly touted the drug.
The team behind the review said, “As hospitals around the world have been filled with patients with COVID-19-19, front-line providers remain without effective therapeutic tools to directly combat the disease. Initial anecdotal reports from China led to wide initial acceptance of HCQ [[[[hydroxychloroquine]and to a lesser extent CQ [[[[chloroquine]for many hospitalized patients with COVID-19-19 worldwide.
“As more data becomes available, enthusiasm for these drugs has subsided. Large, well-designed randomized controlled trials are needed to help determine what role, if any, these medications must have in treatment COVID-19-19 advancing. “
The researchers also noted that antimalarial drugs have a “powerful” effect on the body’s immune response. That is why hydroxychloroquine It is used to treat autoimmune disorders like rheumatoid arthritis and lupus. This in part gave rise to the hope that drugs may be useful for COVID-19-19 patients, the reviewers explained. However, the drugs can actually dampen the body’s natural immune response, they explained.
Poznansky saying Newsweek there is no animal or human data to suggest hydroxychloroquine should be used to treat COVID-19-19, and data from new emerging clinical trials suggest that patients with this disease do not benefit from taking the drug.
“The antiviral data for hydroxychloroquine turned out to be weak at best and all had been generated in the test tube,” said Poznansky.
The team wrote: “For all these reasons, and in the context of accumulation preclinical and clinical data, we recommend that HCQ [[[[hydroxychloroquine]only used for COVID-19-19 in the context of a carefully constructed randomized clinical trial.
“If this agent is used outside of a clinical trial, the risks and benefits should be carefully weighed on a case-by-case basis and reviewed in light of both virus-induced immune dysfunction and a knowntiviral and immune modulating actions of hydroxychloroquine“
YURI CORTEZ / AFP via Getty Images
Poznansky, who is also an infectious disease medicine treating physician at Massachusetts General Hospital, said he wanted to review the safety and efficacy of the medicines after seeing many patients with moderate and severe forms of COVID-19-19 were in hydroxychloroquine “They were not doing well for various reasons.” He wanted to see if the scientific literature could help explain the role that drugs might be playing.
Considering how the treatment of patients with COVID-19 should be addressed, Poznansky said that researchers should carefully analyze the data on pre-existing drugs considered to treat the disease “to be doubly sure” that there is no indication that are ineffective or pose a risk.
Poznansky He said the article is a warning note against instituting the wholesale use of untested or untested drugs “in a completely new infection that is currently incompletely understood. Always remembering that in the beginning in medicine, you don’t do harm. “
“We know that the medical history is riddled with administered medical treatments, popularized in the public and medical spheres, which ultimately did nothing good or worse, were shown to harm.”
He added: “There has never been a more important moment than during this COVID-19-19 pandemic to remember and emphasize the importance of clinical trials to guide clinical decision-making and to emphasize the principles that govern it, including respect for people, charity and justice. “
Andrew Preston, microbial reader Pathogenesis at the University of Bath, UK, who did not work on the review, said Newsweek oralone There have been some controlled trials on the use hydroxychloroquine and he also believed that “these have not yet shown any clear therapeutic effect.
“I think initially much of the use was based on it’s been used for decades on people and therefore it’s safe, so ‘it could do a good thing, it won’t do any harm.’ However, one of The things that will come out of the trials is that it does cause adverse effects, and some of these can be serious, “he said.
Preston added: “There are other large controlled trials underway. They should start producing data soon. Then we will have a much better idea of any therapeutic effects.”
Advice from the Centers for Disease Control and Prevention on the use of facial covers to reduce the spread COVID-19-19
- The CDC recommends using a cloth face covering in public where social distancing measures are difficult to maintain.
- A simple cloth face covering can help delay the spread of the virus by those infected and by those without symptoms.
- Fabric face covers can be made from household items. CDC offers guidelines. (https: //www.Centers for Disease Control and Prevention.gov /coronavirus/ 2019-2019-nCoV/ prevent-get sick /DIY-Fabric coverings for the face.html)
- Fabric face linings should be washed regularly. A washing machine will suffice.
- Practice safe removal of facial covers by not touching your eyes, nose, and mouth, and wash your hands immediately after removing the cover.
Tips from the World Health Organization to prevent the spread of coronavirus disease (COVID-19-19)
Hygiene tips
- Wash your hands frequently with soap and water, or with an alcohol-based hand sanitizer.
- Wash hands after coughing or sneezing; when caring for the sick; before, during and after food preparation; before eating; after using the bathroom; when the hands are visibly dirty; and after handling animals or waste.
- Keep at least 1 meter (3 feet) away from anyone who is coughing or sneezing.
- Avoid touching your hands, nose and mouth. Do not spit in public.
- Cover your mouth and nose with a folded or disposable tissue when you cough or sneeze. Discard the tissue immediately and clean your hands.
Medical advice
- Avoid close contact with other people if you have any symptoms.
- Stay home if you don’t feel well, even with mild symptoms, such as a headache and a runny nose, to prevent the possible spread of the disease to medical facilities and others.
- If you develop severe symptoms (fever, cough, shortness of breath) seek medical attention in advance and contact local health authorities in advance.
- Please note any recent contact with others and travel details to provide authorities that can track and prevent the spread of the disease.
- Stay updated on COVID-19-19 developments issued by the health authorities and follow their guidelines.
Use of mask and gloves
- Healthy people only need to wear a mask if they care for a sick person.
- Wear a mask if you are coughing or sneezing.
- Masks are effective when used in combination with frequent hand cleaning.
- Do not touch the mask while wearing it. Wash your hands if you touch the mask.
- Learn how to properly put on, take off, and dispose of masks. Wash your hands after disposing of the mask.
- Do not reuse disposable skins.
- Regularly washing bare hands is more effective against catching COVID-19-19 need to wear rubber gloves.
- the COVID-19The -19 virus can still be detected with rubber gloves and spread by touching your face.