
Updated: October 29, 2020 12:09:08 am
 A healthcare worker takes a nasal swab sample at a COVID-19 testing center in Hyderabad (AP)
A healthcare worker takes a nasal swab sample at a COVID-19 testing center in Hyderabad (AP)
With the results of late-stage clinical trials by two Covid-19 vaccine pioneers, Moderna Inc and Pfizer, expected in the coming weeks, hopes of securing an antidote to the novel coronavirus in December have risen, a as infections increase in the United States and Europe with the onset of winter. Furthermore, despite minor setbacks during phase III trials in the past two months, the vaccine developed by Oxford University and AstraZeneca Plc have produced immune responses both in the elderly and in the young.
However, the unprecedented rate at which Covid-19 vaccines are being developed (it can typically take 10 to 15 years to bring a vaccine to market) has led experts, including the UK Vaccine Task Force, to say that the the first generation of shots “is probably imperfect” and that “it might not work for everyone.”
According to the World Health Organization (WHO), more than 150 Covid-19 vaccines are currently being developed, with around 44 candidates in clinical trials and 11 undergoing late-stage testing.
Oxford-AstraZeneca Coronavirus Vaccine
In encouraging news, AstraZeneca said earlier this week that the AZD1222 or ChAdOx1 nCoV-19 vaccine candidate “has produced a robust immune response in older adults and the elderly, those most at risk for severe disease.” The development is promising, as older patients have been hit the hardest by Covid-19, with the majority of deaths occurring in those over 60, Bloomberg reported.
Trial participants aged 56 years and older showed low levels of adverse reactions. “It is encouraging to see that immunogenicity responses were similar between older and younger adults and that reactogenicity was lower in older adults, where the severity of Covid-19 disease is higher,” AstraZeneca said.
While AstraZeneca aims to launch the vaccine, made from a weakened version of a common cold virus that causes infections in chimpanzees, by the end of the year, staff at a London hospital have been told to be ready to receive the first batches of the vaccine. Oxford filmed on November 2, The Sun newspaper reported.
In India, the vaccine, which is being tested by the Serum Institute and called Covishield, can be ready in december, with the first batch of 100 million doses available for the second or third quarter of 2021, SII CEO Adar Poonawalla told NDTV.
This occurs even as late-stage injection testing was restarted in the US last week, after it was halted since 9 September after one of the participants developed an “unexplained illness” in the United Kingdom. Earlier this month a volunteer participating in an Oxford vaccine clinical trial died, which raised concern among the scientific community. However, AstraZeneca later said that the participant did not receive the injection from the company.
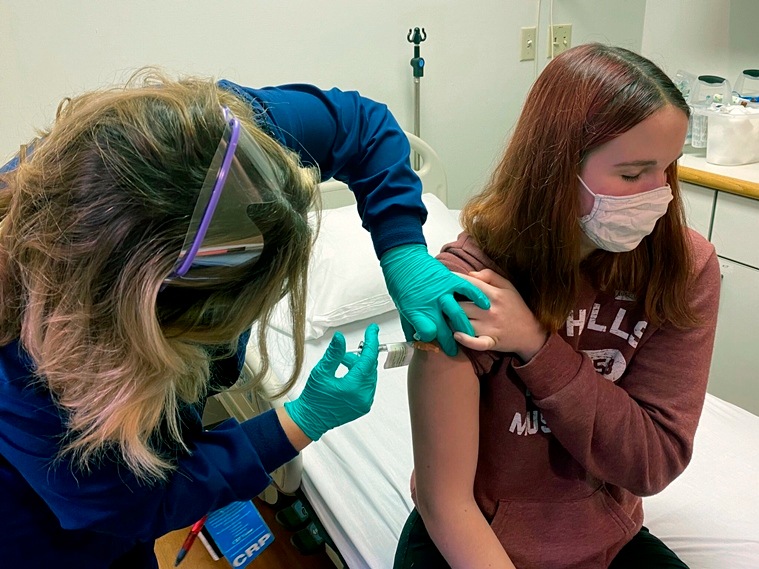 In this photo provided by Cincinnati Children’s Hospital Medical Center, a clinical research coordinator administers an injection to a trial participant as part of Pfizer’s (AP) COVID-19 vaccine hospital clinical trial.
In this photo provided by Cincinnati Children’s Hospital Medical Center, a clinical research coordinator administers an injection to a trial participant as part of Pfizer’s (AP) COVID-19 vaccine hospital clinical trial.
Pfizer coronavirus vaccine
Pfizer, which had previously said it could have efficacy data for the vaccine in October, expressed hope that it could supply about 40 million doses in the United States this year if clinical trials progress as expected and regulators approve its messenger RNA. modified with a single nucleoside (modRNA). vaccine.
“We have reached the last mile here. If all goes well, we will be ready to distribute an initial quantity of doses ”, quoted AFP to the executive president of Pfizer, Albert Bourla. Bourla said the firm had not yet reached key benchmarks for evaluating the vaccine’s efficacy.
Explained | Why the storage and distribution of Covid-19 vaccines will be the next big challenge
Modern vaccine against coronavirus
The US-based Moderna Inc, which is expected to present interim results of its trials of the mRNA-1273 vaccine next month, said a positive result could see the firm gain US approval. For emergency use authorization from December, according to a report in the Wall Street Journal. Moderna intends to produce 20 million doses of its experimental vaccine by the end of the year.
Additionally, in a bid for faster approval, Moderna has started submitting ongoing data on its candidate vaccine to the UK health regulator to begin its independent evaluation of the evidence as it becomes available. Moderna has also requested a similar real-time review of its vaccine in Canada.
Johnson & Johnson Coronavirus Vaccine
Johnson & Johnson, the largest US pharmaceutical company, which has resumed late-stage trials of its single-shot JNJ-78436735 in the United States. after a hiatus due to security issues, recently announced that the first batches of its candidate could be available for emergency use starting in January. Initial results of the 60,000-person study are expected to be available by the end of the year. On October 18, trials of Janssen’s candidate vaccine, which uses a modified adenovirus such as the Oxford injection, were put on hold after one participant developed “unexplained illness.”
Sputnik V coronavirus vaccine
Almost two months after becoming the first country to approve a Covid-19 vaccine amid skepticism from the scientific community, Russia has submitted requests to the WHO for the emergency use list (EUL) and prequalification of its Sputnik vaccine. V. A prequalification assent will make the Russian vaccine eligible for global access if it meets WHO quality, safety and efficacy standards.
Last week, Dr Reddy’s Laboratories (DRL) received regulatory approval from the Comptroller General of Medicines of India to conduct intermediate to late stage human trials of the Sputnik V candidate vaccine in India. Delhi-based Mankind Pharma is likely to market and distribute the Russian Covid-19 vaccine. The vaccine will be tested in around 1,500 participants at at least 10 sites, including those in Maharashtra, Andhra Pradesh and Telangana.
© IE Online Media Services Pvt Ltd
.
 The Indian Express is now on Telegram. Click here to join our channel (@indianexpress) and stay updated with the latest headlines
The Indian Express is now on Telegram. Click here to join our channel (@indianexpress) and stay updated with the latest headlines