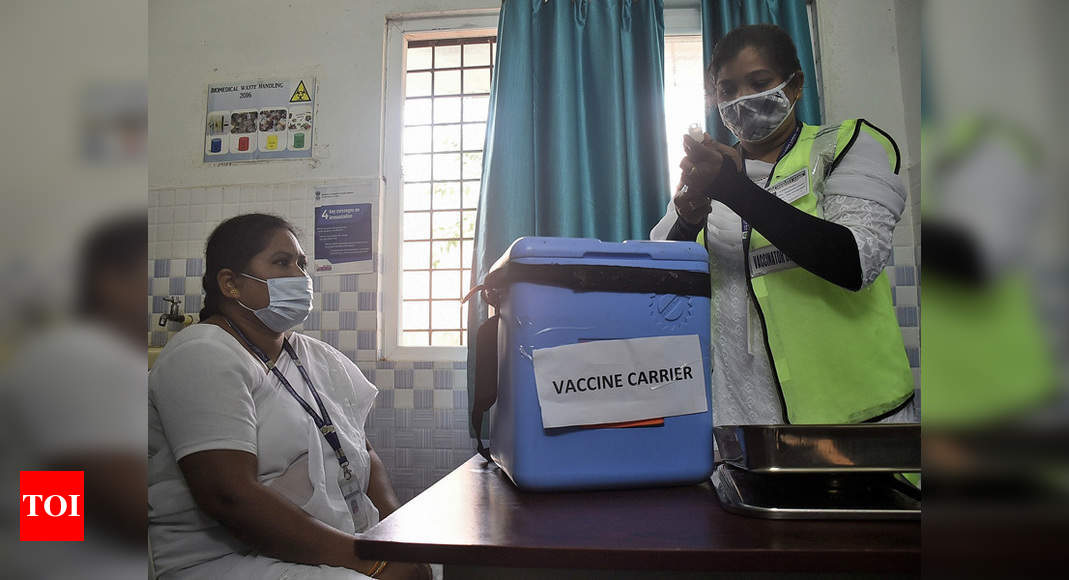
NEW DELHI: A day before the nationwide Covid-19 vaccination trial, the Indian drug regulator’s expert panel on Friday cleared the platforms for emergency use authorization for the vaccine developed by AstraZeneca and the University from Oxford.
The vaccine, which is being produced locally under the name Covishield by the Serum Institute of India, will now receive the final go-ahead from the Comptroller General of Drugs of India (DCGI).
A senior official told TOI that once Covishield’s approval is obtained, the vaccination process is likely to begin in another 7 to 10 days.
National drill
With Covishield just one step away from approval and a couple of others under regulatory review, India is gearing up for a mega-trial on January 2 to test the operational feasibility of its vaccination program.
The activity is proposed to take place in all state capitals at at least 3 session sites.
“The aim of the trial for the introduction of the Covid-19 vaccine is to assess the operational feasibility in using the Co-WIN application in the field setting, test the links between planning and implementation, and identify challenges and guide the way to go before This is also expected to give confidence to program managers at various levels, “said the Union Health Ministry.
Some states will also include districts that are located in difficult terrain / have poor logistical support, while Maharashtra and Kerala will likely schedule the drill in major cities in addition to their capital.
As for the simulation to be carried out, for each of the three session sites, the doctor in charge will identify 25 beneficiaries of the test (health workers).
States and UT have been asked to ensure that the data for these beneficiaries is uploaded to Co-WIN. These beneficiaries will also be available on the session site for the drill. The states and UT will prepare the facilities and users to be created in the Co-WIN app, including uploading the beneficiary data of healthcare workers (HCW), the ministry said.
States and UT have been asked to ensure physical verification of all proposed sites to determine suitability of space, logistical arrangements, Internet connectivity, electricity, security, etc. and prepare at least three model session sites in each state (in the state capital) for the demonstration.
They have been asked to ensure that the model sites have separate entrances and exits in a ‘three-room configuration’ with adequate outdoor space for awareness-raising activities, display all IEC material at these sites, and ensure that that all standard operating procedures and protocols are being practiced at the identified sites in an ideal environment together with vaccination teams to be identified and trained in all aspects.
The trial will also equip the state and UT administration in managing vaccine supply, storage and logistics, including cold chain management, the ministry said.
1 lakh army ready to administer vaccine
Since vaccine administrators will play an important role in the vaccination process, training of trainers and those who will administer the vaccine has begun in several states.
Around 96,000 vaccinators have been trained for the Covid vaccination program that the government plans to implement in January once a vaccine is approved.
State governments, district officials and municipal commissioners have been asked to set up ground staff, logistics and IT systems to carry out the campaign that aims to cover 30 million million priority residents by July.
Vardhan reviews test preparation
Meanwhile, Union Health Minister Harsh Vardhan on Friday chaired a high-level meeting to review readiness at various session sites across the country for the coronavirus vaccination trial to be held on January 2.
Senior officials in the Ministry of Health informed the minister about various improvements that have been made to make India’s dry run fault free, such as the number of telephone operators that has been increased to answer all possible inquiries from the teams in the field performing dry running. to run.
“Working groups have been set up at the block level for the physical inspection of the sites and all workers have been oriented to that effect with the dissemination of frequently asked questions about the process, among other topics,” the ministry said in a statement.
Vardhan asked concerned officials to ensure that vaccination sites and the officer in charge comply with the detailed checklist and SOP for vaccination that has been prepared by the Ministry of Health and shared with the states / UT to guide them through the rehearsal.
He also insisted on the need for a perfect calibration between medical and administrative officials to make the event a manual that would later allow the massive implementation of the vaccination campaign, according to the statement.
Re-emphasizing the importance of an event of this type that involves massive participation similar to elections, the Health Minister said: “Let’s try to implement it as a real exercise with attention to the smallest detail. Proper coordination will go a long way towards creating mutual understanding so that the next vaccination campaign can continue without any problems. ”
Building on the 1994 polio pulse campaign in Delhi, Vardhan said that since the vaccination exercise is integrally based on the interaction and participation of people, there is a need to mobilize relevant stakeholders, NGOs , civil society organizations (CSOs) and others, he said.
He also emphasized the need for adequate security arrangements at session venues, cold chain points and during vaccine transport.
(With PTI inputs)
The vaccine, which is being produced locally under the name Covishield by the Serum Institute of India, will now receive the final go-ahead from the Comptroller General of Drugs of India (DCGI).
A senior official told TOI that once Covishield’s approval is obtained, the vaccination process is likely to begin in another 7 to 10 days.
National drill
With Covishield just one step away from approval and a couple of others under regulatory review, India is gearing up for a mega-trial on January 2 to test the operational feasibility of its vaccination program.
The activity is proposed to take place in all state capitals at at least 3 session sites.
“The aim of the trial for the introduction of the Covid-19 vaccine is to assess the operational feasibility in using the Co-WIN application in the field setting, test the links between planning and implementation, and identify challenges and guide the way to go before This is also expected to give confidence to program managers at various levels, “said the Union Health Ministry.
Some states will also include districts that are located in difficult terrain / have poor logistical support, while Maharashtra and Kerala will likely schedule the drill in major cities in addition to their capital.
As for the simulation to be carried out, for each of the three session sites, the doctor in charge will identify 25 beneficiaries of the test (health workers).
States and UT have been asked to ensure that the data for these beneficiaries is uploaded to Co-WIN. These beneficiaries will also be available on the session site for the drill. The states and UT will prepare the facilities and users to be created in the Co-WIN app, including uploading the beneficiary data of healthcare workers (HCW), the ministry said.
States and UT have been asked to ensure physical verification of all proposed sites to determine suitability of space, logistical arrangements, Internet connectivity, electricity, security, etc. and prepare at least three model session sites in each state (in the state capital) for the demonstration.
They have been asked to ensure that the model sites have separate entrances and exits in a ‘three-room configuration’ with adequate outdoor space for awareness-raising activities, display all IEC material at these sites, and ensure that that all standard operating procedures and protocols are being practiced at the identified sites in an ideal environment together with vaccination teams to be identified and trained in all aspects.
The trial will also equip the state and UT administration in managing vaccine supply, storage and logistics, including cold chain management, the ministry said.
1 lakh army ready to administer vaccine
Since vaccine administrators will play an important role in the vaccination process, training of trainers and those who will administer the vaccine has begun in several states.
Around 96,000 vaccinators have been trained for the Covid vaccination program that the government plans to implement in January once a vaccine is approved.
State governments, district officials and municipal commissioners have been asked to set up ground staff, logistics and IT systems to carry out the campaign that aims to cover 30 million million priority residents by July.
Vardhan reviews test preparation
Meanwhile, Union Health Minister Harsh Vardhan on Friday chaired a high-level meeting to review readiness at various session sites across the country for the coronavirus vaccination trial to be held on January 2.
Senior officials in the Ministry of Health informed the minister about various improvements that have been made to make India’s dry run fault free, such as the number of telephone operators that has been increased to answer all possible inquiries from the teams in the field performing dry running. to run.
“Working groups have been set up at the block level for the physical inspection of the sites and all workers have been oriented to that effect with the dissemination of frequently asked questions about the process, among other topics,” the ministry said in a statement.
Vardhan asked concerned officials to ensure that vaccination sites and the officer in charge comply with the detailed checklist and SOP for vaccination that has been prepared by the Ministry of Health and shared with the states / UT to guide them through the rehearsal.
He also insisted on the need for a perfect calibration between medical and administrative officials to make the event a manual that would later allow the massive implementation of the vaccination campaign, according to the statement.
Re-emphasizing the importance of an event of this type that involves massive participation similar to elections, the Health Minister said: “Let’s try to implement it as a real exercise with attention to the smallest detail. Proper coordination will go a long way towards creating mutual understanding so that the next vaccination campaign can continue without any problems. ”
Building on the 1994 polio pulse campaign in Delhi, Vardhan said that since the vaccination exercise is integrally based on the interaction and participation of people, there is a need to mobilize relevant stakeholders, NGOs , civil society organizations (CSOs) and others, he said.
He also emphasized the need for adequate security arrangements at session venues, cold chain points and during vaccine transport.
(With PTI inputs)
.