[ad_1]
According to sources, ICMR will finance the trials for about 20 health centers for up to a month. “The protocols were developed by ICMR. We called the entrances of hospitals involved in Covid-19 treatment to participate in clinical trials, ”a senior ICMR official, who did not wish to be identified, told ET. “We have received 150 odd requests. The project would finance 20 health centers. “The sources said ICMR wants to start clinical trials in government hospitals to avoid ethics-related problems. The first facility identified is the Ahmedabad-based Sardar Vallabhbhai Patel Hospital, a super government hospital specialized 1,500 beds.
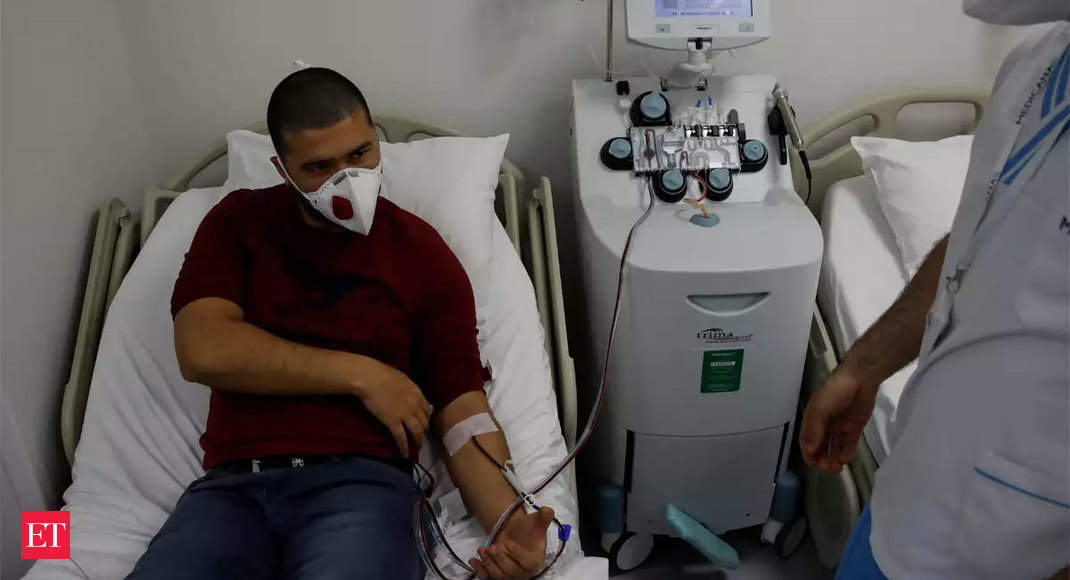
The therapy involves drawing blood from a patient who has recovered a minimum of two weeks from Covid-19. The plasma is then separated from the blood. Approximately 400 ml of plasma can be used to treat two patients. The therapy is based on the fact that the antibodies are generated in a patient suffering from a disease. These antibodies are highest in a person 2-3 weeks after recovery. In the case of Covid-19, the person is considered recovered once they receive two negative reports of the RT-PCR test. Plasma is withdrawn two weeks after the second negative test.
After being chosen for clinical trials of therapy, each healthcare facility will sign a memorandum of understanding with ICMR, which will clearly explain ethical issues, including proper consent for trials and how to search for donors. After this, hospitals will call cured patients to donate blood voluntarily for trials. There are conditions that the hospital must meet. A donor will need to undergo tests to make sure they don’t have hepatitis C and HIV. If considered successful, therapy will not be prohibitively priced in a private hospital.
“These are new treatments in which the hospital would charge for the actual services used. Therefore, it would not cost more than Rs 10-12,000, ”said a spokesman for a private hospital in Delhi. Even before clinical trials began, Delhi-based Max Healthcare had used the therapy on a critically ill patient, who was on a ventilator. After the family obtained a plasma donor, the hospital decided to use the therapy under ‘not indicated use’ and a go-ahead from the hospital’s ethics committee.
[ad_2]