
Updated: June 23, 2020 1:11:52 pm
 Lab technician at Johnson Pharmaceutical, a subsidiary of Johnson & Johnson, during research on coronavirus in Beans, Belgium. (AP photo)
Lab technician at Johnson Pharmaceutical, a subsidiary of Johnson & Johnson, during research on coronavirus in Beans, Belgium. (AP photo)
Coronavirus (Kovid-19) vaccine latest update: Even as the Kovid-19 pandemic has set an unprecedented response among the global scientific community to find the vaccine, the race is set to kick up a notch Next month as three candidates – developed by Modern Inc., China’s Synovac Biotech and UK’s Oxford-AstraZeneca – are set to enter late-stage trials.
According to the latest calculations by the World Health Organization, 13 experimental vaccines are being tested in humans and more than 120 others are in the first stages of development, while the infection is close to 9 million, including 468,484 deaths. China has six candidates under human test, the maximum.
If all goes well, we may have a vaccine for emergency use by November, although experts have said that obtaining regulatory approval after mass production and supply chain issues could push growth into the next year .
The World Health Organization (WHO) Solidarity Trial, the UK’s RECOVERY Trial and the US ‘Operation Warp Motion’ are three of the major scientific efforts in research in the fight against Covid-19.
What are the stages of development of stages vaccine?
First, a new vaccine candidate has to pass the test in animals, followed by a clinical trial. Then in three stages, the safety and efficacy of the vaccine candidate are tested according to protocol. The fourth phase involves the collection and analysis of post-marketing data.
Pre-clinical testing: In this primary phase, scientists test the vaccine on animals such as mice or monkeys to see if it produces an immune response.
Phase I Testing: This is the first stage where the experimental vaccine is given to humans, typically between 20–80 subjects, to test safety and dosage and to test whether it stimulates the immune system.
Phase II test: In this phase, a large group of several hundred individuals is enrolled for testing and is divided into age-groups such as children and the elderly. Phase II testing studies the candidate’s vaccine safety, immunogenicity, proposed dose, schedule of vaccination, and method of delivery.
Phase III test: Since small groups of humans tested in the first phase may not have some side effects, thousands of people are given vaccine candidates in this phase. Here, scientists test how infected people get compared to volunteers receiving a placebo. These tests can determine whether the vaccine protects against coronovirus.
Approval: After the third phase of trials, the vaccine developer submits a license application to the regulatory authority in their respective country. The regulator then inspects the factory where the vaccine will be made and its labeling approved.
During an epidemic, a vaccine may receive emergency use authorization before a formal green signal.
![]()
What type of Kovid-19 vaccines are being developed?
With limited time in their hands, scientists are developing some vaccines from scratch and some from existing molecules developed for other diseases. Based on research, the fact has emerged that the novel Coronavirus is from a family that is already being worked around the world after SARS and MERS.
Genetic vaccines: These are vaccines that use genes from the coronavirus (in the form of DNA or RNA) to provoke an immune response.
Viral vector vaccines: These vaccines use a virus to transmit the coronovirus gene into cells and provoke an immune response. These viruses become weak so they cannot cause disease.
Protein-based vaccines: These vaccines use a coronavirus protein or a protein fragment to provoke an immune response by mimicking the outer coat of the coronovirus.
Whole virus vaccines: Such vaccines use a weak or inactive version of the virus. These vaccines are made by inactivating a pathogen, usually using heat or chemicals. It destroys the infectivity of the pathogen while maintaining its pathogenicity.
 Coronavirus (Kovid-19) vaccine latest update: French President Emmanuel Macron listens to a researcher as he visits an industrial development laboratory at the Sanofi Pasteur plant, the vaccine unit of the French drugmaker. (AP photo)
Coronavirus (Kovid-19) vaccine latest update: French President Emmanuel Macron listens to a researcher as he visits an industrial development laboratory at the Sanofi Pasteur plant, the vaccine unit of the French drugmaker. (AP photo)
 Which Kovid-19 vaccines are the top contenders and keep the promise?
Which Kovid-19 vaccines are the top contenders and keep the promise?
Even though the majority of the more than 120 vaccines under development are undergoing Phase II clinical trials, only two of them are in the combined Phase II / III trials.
# 1. Oxford-AstraZeneca Vaccine (Phase II / III)
The AZD1222 vaccine, being developed jointly by the British-Swedish company AstraZeneca and the University of Oxford, is undergoing phase II / III trials in the UK and Brazil, based on a chimpanzee adenovirus called ChAdOx1. AstraZeneca has already begun mass production of the vaccine candidate and plans to take two billion doses of the coronovirus vaccine in September.
The firm has signed deals to produce 400 million for the US and 100 million for the UK if successful in human trials.
# 2. Modern Commentary (Phase II)
The American firm Modern Inc. is developing a US ‘Operation Tana Speed’ vaccine to produce viral proteins using vaccine RNA. The final phase trial of the MRNA-1273 vaccine is scheduled to begin next month at 30,000 people and the firm expects the vaccine dose to be ready in early 2021.
Earlier this month, preliminary information showed that a series of studies in mice have given some assurance that it may not increase the risk of more severe disease and that a single dose may provide protection against novel coronaviruses .
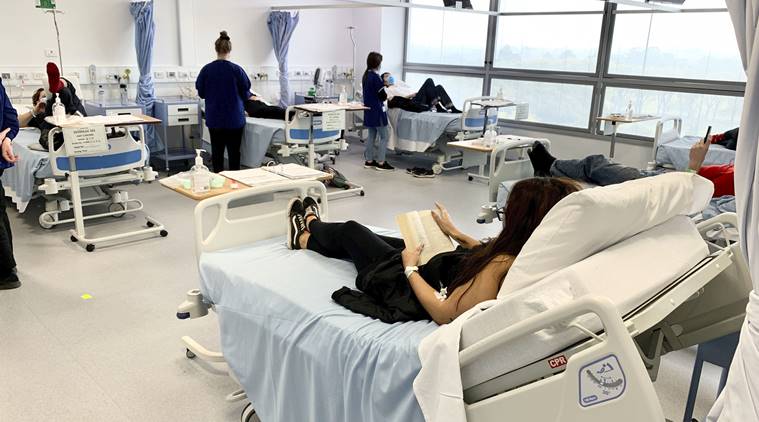 Coronavirus (Kovid-19) vaccine latest update: A coronavirus vaccine is given to clinical trial participants in Melbourne, Australia. (AP)
Coronavirus (Kovid-19) vaccine latest update: A coronavirus vaccine is given to clinical trial participants in Melbourne, Australia. (AP)
# 3. Pfizer-BNTec Vaccine (Phase II)
Pharmaceutical giant Pfizer, which is co-producing the Kovid-19 vaccine with the help of German company BNTECH, has started the dosing process for patients. Four vaccine candidates based on the messenger RNA (mRNA) format are being tested on volunteers. Trials are still underway in Germany and parts of the US. Pfizer believes the Kovid-19 vaccine may be ready by the end of October 2020.
# 4. Imperial College London Vaccine (Phase II)
The vaccine candidate developed by researchers at Imperial College London is based on self-amplified RNA technology and immunized more than 300 healthy people with two doses. The vaccine has been backed by £ 41 million in government funding. Another trial is planned to include 6,000 people for October and if these prove successful, Imperial hopes that the vaccine can be delivered in the UK and overseas early next year.
# 5. Synovac biotech vaccine (second stage)
The Beijing-based company is testing an inactivated vaccine called Coronavac and is preparing for Phase III trials in China and Brazil. Preliminary findings from Phase I and II trials in China showed that its shot is safe and capable of eliciting an immune response from test trials. Synovac’s research and development subsidiary received $ 15 million from private equity firms Advantech Capital and Vivo Capital to finance the development of the vaccine.
# 6. CanSino Biologics (Phase II)
The Chinese vaccine company Cansino Biologics Inc has claimed in a paper in The Lancet that its vaccine candidate based on an adenovirus called Ad5 was capable of producing an immune response against the virus. However, according to the study, approximately 81 percent of all participants showed at least one adverse reaction to the vaccine within the first seven days.
Glenmark announced the launch of favipirvir for the treatment of Kovid patients
# 7. Novavax Vaccine (Phase II)
US biotechnology company Novavax has killed human trials of its protein-based NVX-CoV2373 vaccine for Kovid-19 in Australia. Results of the first phase of clinical trials in Melbourne and Brisbane are expected to be known in July, after which thousands of candidates will again join the second phase in many countries.
Animal testing suggested that the recombinant vaccine is effective at low doses. Novavax plans to manufacture at least 100 million doses this year and 1.5 billion in 2021.
# 8. Cyanoform Vaccine (Phase II)
The China National Biotech Group (CNBG), popularly known as Sinopharm, plans to have human trials abroad by next month. It recently stated that its experimental coronavirus vaccine triggered antibodies in an April clinical trial involving 1,120 healthy participants.
# 9. CureVac Vaccine (Phase I)
German biotech firm CureVac has begun human trials of its coronavirus vaccines involving 168 healthy volunteers. The firm is using a new technology based on mRNA, a type of genetic material never before used to make vaccines.
Earlier this month, the German government said it was spending 300 million euros to take a 23 percent stake in the firm.
# 10. Johnson & Johnson Vaccine (Phase I)
American firm Johnson & Johnson has fast-tracked the start of human clinical trials for its recombinant Ad26.COV2-S vaccine in the second half of July. The company is also beginning negotiations with the National Institute of Allergy and Infectious Diseases (NIAID) to begin a large, late-stage trial ahead of time. In March, J&J signed an agreement with the US government to build enough manufacturing capacity to produce more than 1 billion doses of its vaccines through 2021, Reuters reported.
4 The Indian Express is now on Telegram. Click here to join our channel (@indianexpress) and stay updated with the latest headlines.
For all the latest explained news, download the Indian Express app.
© IE Online Media Services Pvt Ltd
.