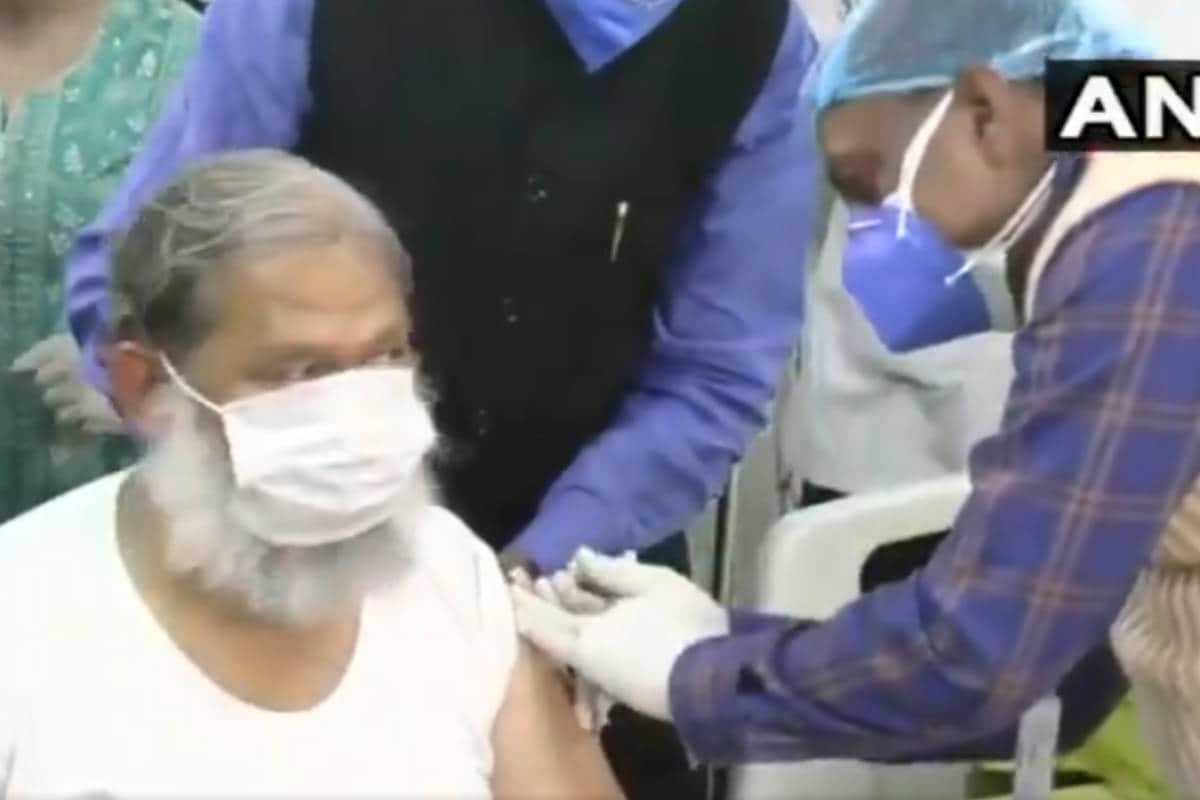

Haryana Health Minister Anil Vij receives a test dose of Covaxin, Bharat Biotech’s Covid-19 vaccine candidate, at Ambala Cantt Civil Hospital on Friday. (Twitter / @ ANI)
The 67-year-old senior BJP leader was dosed under the supervision of a team of doctors from PGI Rohtak and the Department of Health.
- News18.com
- Last update: November 20, 2020 1:08 PM IST
- FOLLOW US:
Haryana Health Minister Anil Vij received a test dose of Bharat Biotech. COVID-19 the Covaxin vaccine candidate at the Ambala Cantt Civil Hospital. Vij had volunteered to be the first participant in the phase three trial that began in the state on Friday.
the Covaxin vaccine candidate at the Ambala Cantt Civil Hospital. Vij had volunteered to be the first participant in the phase three trial that began in the state on Friday.
The 67-year-old senior BJP leader was dosed under the supervision of a team of doctors from PGI Rohtak and the Department of Health.
Vij, who is a member of the Ambala Cantt community, said Wednesday that the third phase of the Covaxin trial will begin in the state on November 20 and has volunteered to be the first volunteer to be vaccinated.
Covaxin is being indigenously developed by Bharat Biotech in collaboration with the Indian Council for Medical Research (ICMR). Last month, the vaccine manufacturer said it had successfully completed the interim analysis of the phase 1 and 2 trials and is starting phase 3 trials.
Bharat Biotech had said on Monday that Covaxin’s phase 3 trial will involve 26,000 volunteers at 25 centers in India and is being carried out in partnership with the ICMR. It is the largest clinical trial conducted for a COVID-19 vaccine in India.
vaccine in India.
This is India’s first phase 3 efficacy study for a COVID-19 vaccine and the largest phase 3 efficacy trial ever conducted. Covaxin’s human trial had started in July at the Rohtak Graduate Institute of Medical Sciences, Vij had previously said.
vaccine and the largest phase 3 efficacy trial ever conducted. Covaxin’s human trial had started in July at the Rohtak Graduate Institute of Medical Sciences, Vij had previously said.
.