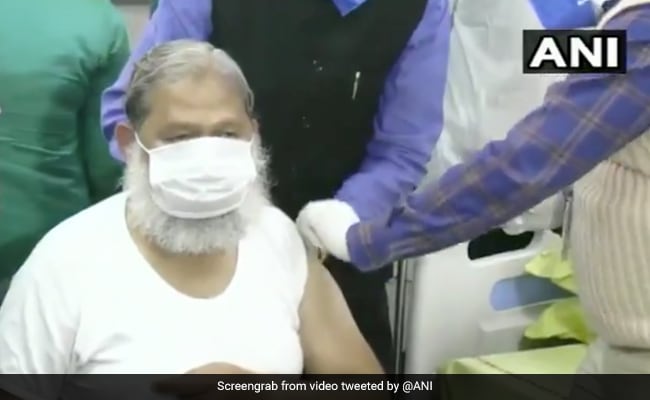
Anil Vij received a test dose of a coronavirus vaccine today at a hospital in Ambala
New Delhi:
Haryana Health Minister Anil Vij received a test dose of a coronavirus vaccine today at a hospital in the state’s Ambala cantonment. The third phase trial of Covaxin from Bharat Biotech started today in Haryana.
Just a few days ago, the 67-year-old minister had announced on Twitter that he will be the first volunteer in his state for the anti-Covid vaccine.
The senior leader of the BJP tweeted on Thursday: “I will be administered a test dose of the Covaxin vaccine for coronavirus, a product of Bharat Biotech, tomorrow at 11 am at the Civil hospital, Ambala Cantt, under the expert supervision of a team of doctors from PGI Rohtak and the Department of Health. “
#CLOCK Haryana Health Minister Anil Vij receives a test dose of #Covaxin, in a hospital in Ambala.
He had volunteered to be the first volunteer for Covaxin’s third-phase trial, which began today in the state. pic.twitter.com/xKuXWLeFAB
– ANI (@ANI) November 20, 2020
Bharat Biotech announced on Monday the start of phase III trials of Covaxin, involving 26,000 volunteers across India. The trials will be carried out in partnership with the Indian Council for Medical Research (ICMR) and will be the largest clinical trial conducted for a COVID-19 vaccine in India, according to the ANI news agency. The trial has been approved by the Comptroller General of Drugs of India.
Covaxin has been evaluated in approximately 1,000 subjects in phase I and phase II clinical trials, with promising safety and immunogenicity data.
In Haryana, the Pandit Bhagwat Dayal Sharma University of Health Sciences in Rohtak and the ESIC hospital in Faridabad have been identified among the sites in India where the trials will take place, according to the statement by Bharat Biotech cited by ANI.

The phase 3 trial of Covaxin will involve 26,000 volunteers at 25 centers in India and is being carried out in partnership with the ICMR, the company said.
This is India’s first phase III efficacy study for a COVID-19 vaccine, and the largest phase III efficacy trial ever conducted.
Covaxin’s human trial had started in July at the Rohtak Graduate Institute of Medical Sciences, Anil Vij had previously said.
.