Updated: October 27, 2020 9:22:04 am
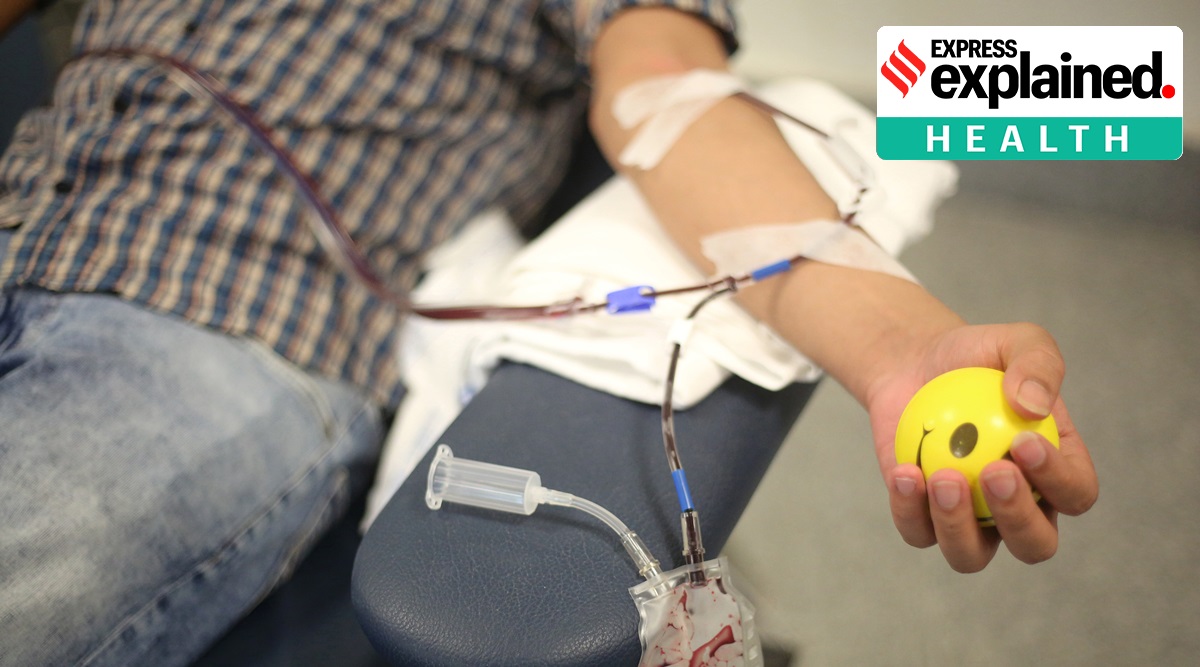
 A 28-year-old man donates his blood plasma for Covid-19 treatment at the Delhi Plasma Bank at the Institute of Liver and Biliary Sciences. (Express photo: Tashi Tobgyal)
A 28-year-old man donates his blood plasma for Covid-19 treatment at the Delhi Plasma Bank at the Institute of Liver and Biliary Sciences. (Express photo: Tashi Tobgyal)
Recently published findings on convalescent plasma therapy in Covid-19 patients have sparked a debate about its efficacy. After the largest trial of this type in the country, known by the acronym PLACID, it was found that convalescent plasma was ineffective In arresting Covid-19, the Indian Council of Medical Research (ICMR) has been considering leaving this option of national guidelines. However, in several states, including Maharashtra and Delhi, the most affected, health authorities continue to push the option, while those who run plasma blood banks promote it with anecdotal accounts on social media.
What is plasma therapy?
Plasma is the liquid part of the blood. Convalescent plasma, taken from the blood of patients recovering from infection, is a source of antibodies against infection. Therapy involves using your plasma to help others recover. For Covid-19, this has been one of the treatment options. The donor would have to be a documented case of Covid-19 and be healthy for 28 days from the last symptoms.
What has happened to provoke the debate?
An ICMR study has found that convalescent plasma was not associated with a reduction in progression to severe Covid-19 or all-cause mortality. Results from the multi-center PLACID trial, first on a prepress server in September, were published in the peer-reviewed British Medical Journal on October 22.
At a recent press conference, ICMR CEO Dr. Balram Bhargava had said that they were contemplating removing convalescent plasma as a definitive therapy from the national guidelines for the treatment of Covid-19.
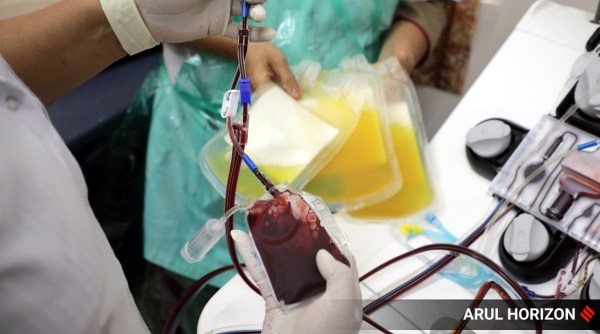 Staff from the Maharashtra State Reserve Police Force donate plasma in a plasma donation drive at Sassoon Hospital in Pune. (Express photo: Arul Horizon)
Staff from the Maharashtra State Reserve Police Force donate plasma in a plasma donation drive at Sassoon Hospital in Pune. (Express photo: Arul Horizon)
What are the results of the trial?
Although the use of convalescent plasma appeared to improve the resolution of shortness of breath and fatigue in patients with moderate Covid-19, this did not translate into a reduction in mortality at 28 days or progression to severe disease .
PLACID was a randomized controlled trial in 39 hospitals spread over 14 states and territories of the Union and representing 25 cities. It covered 464 adults admitted to the hospital (examined from April 22 to July 14) with confirmed moderate Covid-19, of whom 235 were assigned to convalescent plasma therapy, while 229 were randomly assigned to receive the best standard of care. The group of 235 received two 200 ml doses of convalescent plasma, 24 hours apart.
Progression to severe disease or death at 28 days after enrollment occurred in 44 (19%) of the participants in the intervention arm compared with 41 (18%) in the control arm. A higher proportion of patients in the intervention arm showed resolution of shortness of breath and fatigue on day 7, while resolution of fever and cough did not differ between the two arms. “These are clinically significant results,” said Dr. Aparna Mukherjee, principal investigator for the PLACID trial.
Have there been such results elsewhere?
In China, a controlled trial of 103 patients with severe Covid-19 reported no effect of convalescent plasma treatment in terms of time to clinical improvement. In the Netherlands, the ConCOVID trial, which ended prematurely after the enrollment of 86 patients, could find no effect on mortality at 60 days, hospital stay or severity of illness at 15 days.
So who continues to push for convalescent plasma therapy?
Delhi Health Minister Satyendra Jain has said convalescent plasma played an important role in his recovery and many lives have been saved. In Maharashtra, the government has been carrying out the Platina trial on seriously ill patients. It is the brainchild of the Department of Medical Education and CM Uddhav Thackeray.
Dr. Mohammed Faizal, the Platina Project state coordinator, said that at least 40% of the trial has been completed. “We should be able to complete the test in another three months. We have recruited 132 patients and the total target is 472, ”said Dr. Faizal.
Officials said there is no order or letter from ICMR regarding stopping the use of convalescent plasma. “However, ours is a scientific research trial and regardless of the results of the PLACID trial, the PLATINA trial will continue,” said Dr. Faizal. 
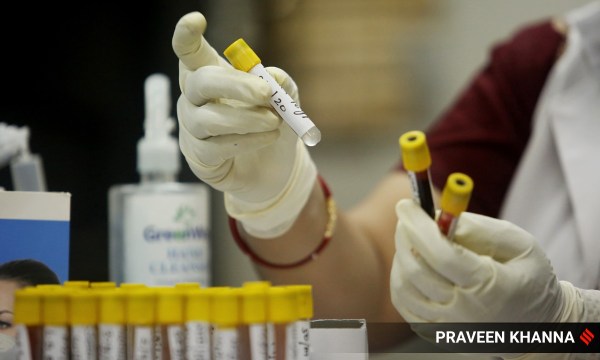 A health worker collects blood samples at a local health center for Covid-19 in New Delhi. (Express photo: Praveen Khanna)
A health worker collects blood samples at a local health center for Covid-19 in New Delhi. (Express photo: Praveen Khanna)
What happens if ICMR removes the therapy from its guidelines?
The ICMR has been cautious due to the trial’s findings. Experts said, however, that the guidelines are not necessarily binding and it is too early to rule out convalescent plasma therapy.
But there are other problems. The authorization of convalescent plasma as a treatment for Covid-19 in India has led to questionable practices, such as calls to donors on social media and the sale of convalescent plasma on the black market. Although convalescent plasma is a safe form of treatment when transfused in accordance with regulations, it involves resource-intensive processes such as plasmapheresis, plasma storage, and measurement of neutralizing antibodies. A limited number of institutes in India have the capacity to carry out these procedures in a quality-assured manner, the BMJ daily said.
So what is the way forward?
This is a new virus, and evidence of the best treatment options is still emerging around the world. For example, remdesivir has been approved as the drug of choice by the US drug regulator, while the World Health Organization’s Solidarity Trial has found it to have little to no effect on Covid mortality at 28 days. And experts said the use of convalescent therapy has saved some lives, but the PLACID trial has raised concerns.
Dr Shashank Joshi, a member of the Maharashtra task force on Covid-19 and dean of the College of Physicians, said that long before the evidence from the trials, the task force had introduced some safeguards to control the indiscriminate use of drugs. like remdesivir. “Covid care is individualized care. Using the right medications in the right patient works. Some of the therapies can be followed with compassion, ”Dr. Joshi said. He said there were limitations to the Solidarity trial, which was not placebo controlled. While the results of the Platina trial are awaited, Dr. Joshi said that the results of one or two trials will not change the outcome of a recommendation.
Don’t miss Explained | School closure, work from home, banning of public events: which works best to restrict Covid-19?
Elizabeth Pathak, president of the Women’s Institute for Independent Social Research, who has written in the BMJ about the trial’s findings, said: “Potential damages from non-immune components of convalescent plasma should be rigorously investigated, only donor plasma with detectable titers of Neutralizing antibodies should be administered to trial participants, to ensure the potential for benefit exists for all patients in the intervention group. “
© The Indian Express (P) Ltd
.