
Although the SII must request emergency use, the company is expected to do so in December. The Center is finalizing a contract with vaccine manufacturers for the acquisition of doses. The government, which will be making bulk purchases, has also negotiated a better price, almost half the likely MRP of Rs 500-600 for the two-shot vaccine, an official source said.
Covaxin from Bharat Biotech may be considered for emergency approval after data from Phase I and II trials are submitted. Regulatory sources told TOI that Bharat Biotech is in the process of releasing data for the vaccine, which is now in phase 3 trials in India. So, two vaccines could be available by February.
“If everything goes according to plan and the company (SII) manages to obtain emergency authorization in December, we expect the first batch of vaccines for January-February and the first group of beneficiaries has been identified,” said the official.
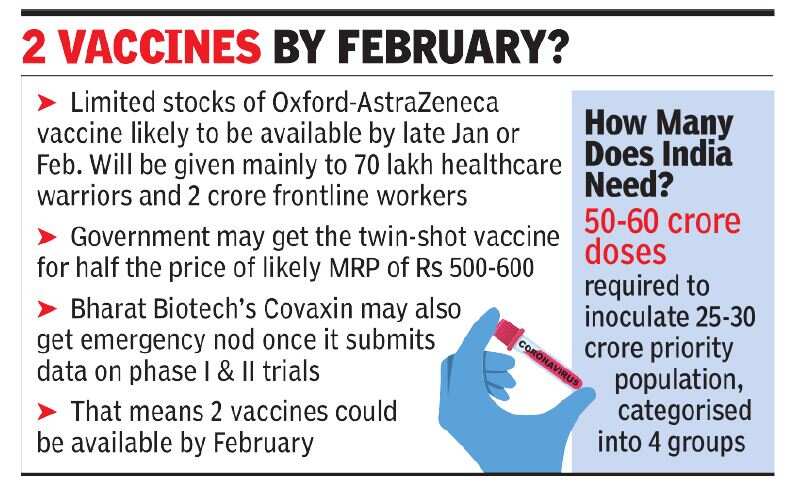
While estimated doses of Rs 50 to 60 million will be needed to inoculate a priority population of Rs 25 to 30 million classified into four different groups, limited reserves are initially expected by the end of January to be administered primarily to some of the 70 lakhs. estimates from healthcare professionals and more. 2 crore of frontline workers, including the police, municipal workers and the armed forces.
IBS has almost completed its phase 3 trials in India and monitoring of the data is likely to begin soon.
“If the Serum Institute sends its efficacy data from the UK and requests an emergency clearance here, it can easily be granted. But even in the case of Bharat Biotech, if the company requests emergency use approval after its phase 1 and phase 2 data is released, the regulator may consider the same, ”the official said, indicating that for February- March, more than one vaccine is likely to obtain at least one emergency use authorization provided the regulator considers the data generated from clinical trials satisfactory.
Meanwhile, the government is formulating terms of reference for two main bodies dealing with the introduction of vaccines, the National Technical Advisory Group on Immunization and the Central Organization for Medicines Standards and Control, to study the granting of an emergency authorization. .
.