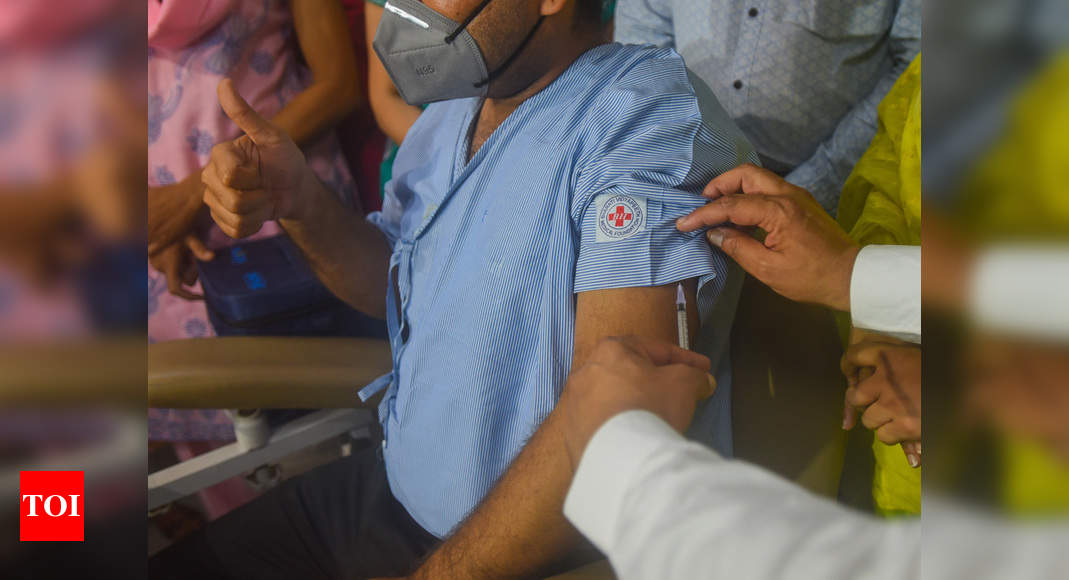
NEW DELHI: The Serum Institute of India said Wednesday that its trials of AstraZeneca’s Covid-19 candidate vaccine in the country were ongoing and had not faced any issues.
The statement came after AstraZeneca Plc stopped injecting its experimental coronavirus vaccine after a person participating in the studies became ill.
“We cannot comment much on the trials in the UK,” Serum Institute India (SII) said in a statement adding “as far as the trials in India are concerned, it continues and we have not faced any problems.”
Serum Institute had partnered manufacturing with AstraZeneca to produce and supply 1 billion doses of the Covid-19 vaccine being developed by the University of Oxford.
The Indian company is conducting clinical trials of the AstraZeneca vaccine candidate in India.
Last month, the Comptroller General of Medicines of India granted permission to the Pune-based SII to conduct phase two and three human clinical trials of the coronavirus vaccine candidate developed by the University of Oxford and the British pharmaceutical firm. Swedish AstraZeneca.
AstraZeneca’s hiatus was due to a standard review of the company’s vaccine trials after a person developed an unexplained illness, the firm said in a statement.
The move was intended to give researchers time to examine safety data while maintaining the integrity of the trials, the British-Swedish company said.
The statement came after AstraZeneca Plc stopped injecting its experimental coronavirus vaccine after a person participating in the studies became ill.
“We cannot comment much on the trials in the UK,” Serum Institute India (SII) said in a statement adding “as far as the trials in India are concerned, it continues and we have not faced any problems.”
Serum Institute had partnered manufacturing with AstraZeneca to produce and supply 1 billion doses of the Covid-19 vaccine being developed by the University of Oxford.
The Indian company is conducting clinical trials of the AstraZeneca vaccine candidate in India.
Last month, the Comptroller General of Medicines of India granted permission to the Pune-based SII to conduct phase two and three human clinical trials of the coronavirus vaccine candidate developed by the University of Oxford and the British pharmaceutical firm. Swedish AstraZeneca.
AstraZeneca’s hiatus was due to a standard review of the company’s vaccine trials after a person developed an unexplained illness, the firm said in a statement.
The move was intended to give researchers time to examine safety data while maintaining the integrity of the trials, the British-Swedish company said.
.