[ad_1]
Updated: May 14, 2020 8:37:52 pm
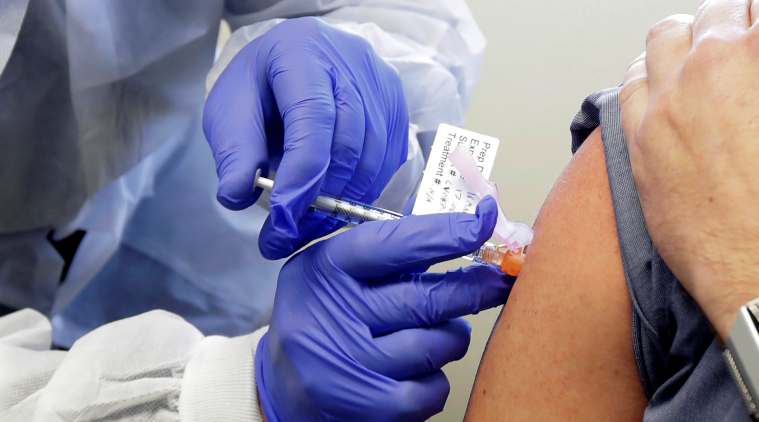
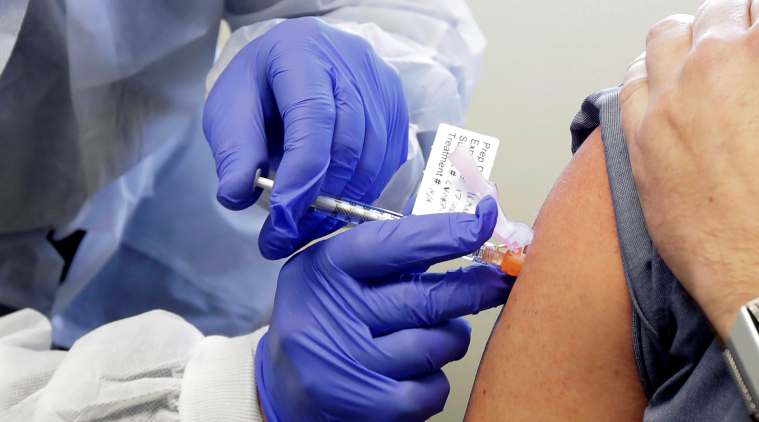 A person receives an injection in the first-stage safety study clinical trial of a potential COVID-19 vaccine. (AP)
A person receives an injection in the first-stage safety study clinical trial of a potential COVID-19 vaccine. (AP)
With scientists and researchers racing against time to find a cure for the new coronavirus, which has infected more than 4.2 million people, this week has seen considerable developments from companies like Moderna and Novavax in the development of a Covid-19 vaccine.
From Moderna Therapeutics obtaining accelerated approval from the United States Food and Drug Administration (FDA) for its Covid-19 vaccine to Novavax to move on to human trials, the world is trying its best to get its hands on a vaccine.
For Covid-19, a vaccine is of paramount importance at this stage, and there are over 100 research groups around the world vying to develop one. These projects are in various stages of development, from research to clinical trials. Vaccines are important because they save a lot of resources to prevent a disease and not have to treat it.
Latest advances in the search for a vaccine for Covid-19:
👉 American biotech company Moderna Therapeutics He said earlier this week that he received “fast approval” from the United States Food and Drug Administration (FDA) for his possible Covid-19 vaccine and that he would conduct his phase 2 trial, according to USA Today.
In the second phase, Moderna will recruit 600 healthy volunteers, half of whom are between 18 and 55 years old and the rest over 55 years old, to test their experimental candidate for the mRNA vaccine, mRNA-1273.
The phase II study will evaluate the safety, reactogenicity, and immunogenicity of two mRNA-1273 vaccines administered 28 days apart. Participants will receive placebo, a dose of 50 µg or 250 µg in both vaccines and will be followed up to 12 months after the second vaccine.
“The Fast Track designation underscores the urgent need for a vaccine against the new coronavirus,” Tal Zaks, MD, PhD, medical director of Moderna, told reporters.
Moderna’s vaccine is based on the virus’s mRNA: it involves injecting fragments of the viral genetic material into the body, which then stimulates the body’s immune system to fight the new coronavirus.
👉 Novavax based in the USA USA, which recently received $ 384 million in funding from the Coalition for epidemic preparedness innovations, said it would begin human testing of its NVX-COV2373 vaccine. The vaccine has shown promising results in animal testing, and its next phase will see testing in 130 humans from Australia.
Dr. Gregory Glenn, president of research and development for Novavax, told the US media that single and double doses of the vaccine had shown great promise in mice and baboons. The vaccine has been designed from a genetic sequence of the SARS-COV-2 virus.
With Novavax’s Matrix-M technology, the vaccine can enhance the immune response and stimulate high levels of neutralizing antibodies.
👉 Hours after affirming that EE. USA He would get the first access to his Covid-19 vaccine, which would provoke a dispute, French pharmaceutical group Sanofi He said he would make it available in all countries. In an interview with Bloomberg, Sanofi CEO Paul Hudson said that “the United States government has the right to the largest pre-order because it has invested in taking the risk.”
The US agency Biomedical Advanced Research and Development Authority has funded the development of the vaccine. However, Hudson’s comments have scorched the French government, with junior economy minister Agnes Pannier-Runacher saying it would be “unacceptable for a country to have privileged access on the pretext of money reasons.”
Sanofi is using an existing technology for its vaccine that was designed for influenza and is applying it to the new virus that causes Covid-19.
👉 Tel Aviv University based in Israel It has partnered with Swiss-based biopharmaceutical company Neovii to develop a vaccine against Covid-19, the Jerusalem Post reported.
Giving an insight into development, Professor Jonathan Gershoni of TAU’s Cell Molecular Biology and Biotechnology Faculty said the goal of the research is to develop a vaccine that could potentially be more efficient and safer than other vaccines due to the ability to target only the specific part of the virus that attacks the cell and not the entire protein.
“This will save a lot of energy to the immune system and avoid possible negative reactions mediated by irrelevant antibodies that have” lost their target “, something that can happen with less focused vaccines,” said Jerusalem Post.
📣 The Indian Express is now on Telegram. Click here to join our channel (@indianexpress) and stay updated with the latest headlines
For the latest news about the coronavirus outbreak, download the Indian Express app.
© IE Online Media Services Pvt Ltd
.
[ad_2]