[ad_1]
[{“available”:true,”c_guid”:”9706200c-aebf-43f0-8fdc-0266fed1459f”,”c_author”:”MTI”,”category”:”vilag”,”description”:”A brazil elnök szerint nemzetközi intézmények hamis információkat terjesztenek az erdőtüzekről.”,”shortLead”:”A brazil elnök szerint nemzetközi intézmények hamis információkat terjesztenek az erdőtüzekről.”,”id”:”20201002_Tovabb_terjed_a_tuz_az_Amazonas_videken_Bolsonaro_a_civileket_es_a_bennszulotteket_okolja”,”image”:”https://img1.hvg.hu/image.aspx?id=9706200c-aebf-43f0-8fdc-0266fed1459f&view=ffdb5e3a-e632-4abc-b367-3d9b3bb5573b”,”index”:0,”item”:”a17ae264-ffc4-4b91-ab2d-72996600e48d”,”keywords”:null,”link”:”/vilag/20201002_Tovabb_terjed_a_tuz_az_Amazonas_videken_Bolsonaro_a_civileket_es_a_bennszulotteket_okolja”,”timestamp”:”2020. október. 02. 07:43″,”title”:”Tovább pusztít a tűz az Amazonas vidékén, Bolsonaro a civileket és a bennszülötteket okolja”,”trackingCode”:”RELATED”,”c_isbrandchannel”:false,”c_isbrandcontent”:false,”c_isbrandstory”:false,”c_isbrandcontentorbrandstory”:false,”c_isbranded”:false,”c_ishvg360article”:false,”c_partnername”:null,”c_partnerlogo”:”00000000-0000-0000-0000-000000000000″,”c_partnertag”:null},{“available”:true,”c_guid”:”f44983e7-83f5-4e72-a5f0-374e166410c4″,”c_author”:”hvg.hu”,”category”:”elet”,”description”:”Sacha Baron Cohen fogalmatlan kazah újságíróként nem győzi dicsérni Donald Trumpot.”,”shortLead”:”Sacha Baron Cohen fogalmatlan kazah újságíróként nem győzi dicsérni Donald Trumpot.”,”id”:”20201001_Borat_altesti_maszkviselessel_provokalva_ter_vissza”,”image”:”https://img1.hvg.hu/image.aspx?id=f44983e7-83f5-4e72-a5f0-374e166410c4&view=ffdb5e3a-e632-4abc-b367-3d9b3bb5573b”,”index”:0,”item”:”7ef4d411-59b4-4a6d-9c68-63c048f4ea4f”,”keywords”:null,”link”:”/elet/20201001_Borat_altesti_maszkviselessel_provokalva_ter_vissza”,”timestamp”:”2020. október. 01. 13:46″,”title”:”Borat altesti maszkviseléssel provokálva tér vissza”,”trackingCode”:”RELATED”,”c_isbrandchannel”:false,”c_isbrandcontent”:false,”c_isbrandstory”:false,”c_isbrandcontentorbrandstory”:false,”c_isbranded”:false,”c_ishvg360article”:false,”c_partnername”:null,”c_partnerlogo”:”00000000-0000-0000-0000-000000000000″,”c_partnertag”:null},{“available”:true,”c_guid”:”94df2f91-2dec-4ee3-ad8e-e2526faf3459″,”c_author”:”hvg.hu”,”category”:”tudomany”,”description”:”A Puli Space Technologies szeretné, ha az Aranycsapat emléke az idők végezetéig fennmaradna, ezért a róluk szóló információkat – egy időkapszulába zárva – a Holdra vinnék.”,”shortLead”:”A Puli Space Technologies szeretné, ha az Aranycsapat emléke az idők végezetéig fennmaradna, ezért a róluk szóló…”,”id”:”20201001_puskas_ferenc_aranycsapat_plakett_idokapszula_hold_puli_space_technologies”,”image”:”https://img1.hvg.hu/image.aspx?id=94df2f91-2dec-4ee3-ad8e-e2526faf3459&view=ffdb5e3a-e632-4abc-b367-3d9b3bb5573b”,”index”:0,”item”:”17936080-a8b2-4c11-9ecc-337952abaf4e”,”keywords”:null,”link”:”/tudomany/20201001_puskas_ferenc_aranycsapat_plakett_idokapszula_hold_puli_space_technologies”,”timestamp”:”2020. október. 01. 18:45″,”title”:”A Holdig vinné Puskást és az egész Aranycsapatot a magyar űrvállalat”,”trackingCode”:”RELATED”,”c_isbrandchannel”:false,”c_isbrandcontent”:false,”c_isbrandstory”:false,”c_isbrandcontentorbrandstory”:false,”c_isbranded”:false,”c_ishvg360article”:false,”c_partnername”:null,”c_partnerlogo”:”00000000-0000-0000-0000-000000000000″,”c_partnertag”:null},{“available”:true,”c_guid”:”c6686b00-295c-49fc-ba7f-ee14a9f8390a”,”c_author”:”Sztojcsev Iván”,”category”:”gazdasag”,”description”:”Nem konkrét korrupciógyanús ügyeket elemez az Európai Bizottság Magyarországról szóló jelentése, hanem azt mutatja be, miért gyenge az állam védekezőképessége a korrupcióval szemben. A magyar kormány úgy tesz, mint ha csakis a közigazgatásban lehetnének megvesztegetések, és azzal, hogy ez ellen mindent megtesz, megpróbálja elpalástolni, mennyire rossz a helyzet minden más téren – állapították meg. A jelentés kilenc pontban mutatja be a magyar korrupció helyzetét, ezeket néztük át.”,”shortLead”:”Nem konkrét korrupciógyanús ügyeket elemez az Európai Bizottság Magyarországról szóló jelentése, hanem azt mutatja be…”,”id”:”20201002_korrupcio_europai_bizottsag_jelentes”,”image”:”https://img1.hvg.hu/image.aspx?id=c6686b00-295c-49fc-ba7f-ee14a9f8390a&view=ffdb5e3a-e632-4abc-b367-3d9b3bb5573b”,”index”:0,”item”:”8624b9d5-4c23-4bb1-b8fd-a17c78918b53″,”keywords”:null,”link”:”/gazdasag/20201002_korrupcio_europai_bizottsag_jelentes”,”timestamp”:”2020. október. 02. 17:00″,”title”:”Ott nem harcol a kormány a korrupció ellen, ahol igazán kellene”,”trackingCode”:”RELATED”,”c_isbrandchannel”:false,”c_isbrandcontent”:false,”c_isbrandstory”:false,”c_isbrandcontentorbrandstory”:false,”c_isbranded”:false,”c_ishvg360article”:false,”c_partnername”:null,”c_partnerlogo”:”00000000-0000-0000-0000-000000000000″,”c_partnertag”:null},{“available”:true,”c_guid”:”56985615-dbb6-4d81-9844-edc942c7bce3″,”c_author”:”MTI”,”category”:”itthon”,”description”:”Már öt magyarországi megyét minősített “vörösnek” a német közegészségügyi intézet. “,”shortLead”:”Már öt magyarországi megyét minősített “vörösnek” a német közegészségügyi intézet. “,”id”:”20201001_Koronavirus_nemetorszag_magyar_megye_kockazatos”,”image”:”https://img1.hvg.hu/image.aspx?id=56985615-dbb6-4d81-9844-edc942c7bce3&view=ffdb5e3a-e632-4abc-b367-3d9b3bb5573b”,”index”:0,”item”:”572c5f5e-689f-4949-827f-e50b6fb80279″,”keywords”:null,”link”:”/itthon/20201001_Koronavirus_nemetorszag_magyar_megye_kockazatos”,”timestamp”:”2020. október. 01. 10:00″,”title”:”A németek újabb három magyar megyét nyilvánítottak kockázatosnak”,”trackingCode”:”RELATED”,”c_isbrandchannel”:false,”c_isbrandcontent”:false,”c_isbrandstory”:false,”c_isbrandcontentorbrandstory”:false,”c_isbranded”:false,”c_ishvg360article”:false,”c_partnername”:null,”c_partnerlogo”:”00000000-0000-0000-0000-000000000000″,”c_partnertag”:null},{“available”:true,”c_guid”:”34d3fcfc-1b97-4d4d-9e7f-63b06fcb9c58″,”c_author”:”hvg.hu”,”category”:”itthon”,”description”:”Belső vizsgálatot indított az óbudai polgármester, miután az egyik kerületi óvónőről kiderült, hogy a rábízott óvodásokról pornográf képeket készített. A vizsgálat után Kiss László polgármester azt állítja, az előző vezetés el akarta tussolni az ügyet. Megkerestük Bús Balázs volt polgármestert, aki azt mondja, a hatóságok kérésnek megfelelően járt el. Közben kiderült, az óvónő és élettársa ellen már vádat emelt az ügyészség, novemberben kezdődik a bírósági eljárás. “,”shortLead”:”Belső vizsgálatot indított az óbudai polgármester, miután az egyik kerületi óvónőről kiderült, hogy a rábízott…”,”id”:”20201001_obuda_gyermekpornografia_kiss_bus”,”image”:”https://img1.hvg.hu/image.aspx?id=34d3fcfc-1b97-4d4d-9e7f-63b06fcb9c58&view=ffdb5e3a-e632-4abc-b367-3d9b3bb5573b”,”index”:0,”item”:”b0e9690a-335a-4068-849a-19bee30d2c55″,”keywords”:null,”link”:”/itthon/20201001_obuda_gyermekpornografia_kiss_bus”,”timestamp”:”2020. október. 01. 18:06″,”title”:”Kiss László szerint az előző óbudai vezetés eltussolt volna egy gyermekpornográf ügyet, Bús Balázs szerint ez nem igaz”,”trackingCode”:”RELATED”,”c_isbrandchannel”:false,”c_isbrandcontent”:false,”c_isbrandstory”:false,”c_isbrandcontentorbrandstory”:false,”c_isbranded”:false,”c_ishvg360article”:false,”c_partnername”:null,”c_partnerlogo”:”00000000-0000-0000-0000-000000000000″,”c_partnertag”:null},{“available”:true,”c_guid”:”62e5772e-290b-4492-8c13-07b98d1cc485″,”c_author”:”HVG”,”category”:”360″,”description”:”„Leaves on the line”, vagyis levelek a sínen. Az angolok ennek hallatán már csak fáradtan nevetnek, mint egy régi kabarétréfán, de az abszurd előadás továbbra is műsoron van. “,”shortLead”:”„Leaves on the line”, vagyis levelek a sínen. Az angolok ennek hallatán már csak fáradtan nevetnek, mint egy régi…”,”id”:”202040_levelek_asinen_a_tannin_hatasa_amenetrendre”,”image”:”https://img1.hvg.hu/image.aspx?id=62e5772e-290b-4492-8c13-07b98d1cc485&view=ffdb5e3a-e632-4abc-b367-3d9b3bb5573b”,”index”:0,”item”:”c8a18cfe-d764-4909-871a-f0be87bf032e”,”keywords”:null,”link”:”/360/202040_levelek_asinen_a_tannin_hatasa_amenetrendre”,”timestamp”:”2020. október. 01. 14:00″,”title”:”Megjött a vasút és a metró aktuális ellensége: az ősz”,”trackingCode”:”RELATED”,”c_isbrandchannel”:false,”c_isbrandcontent”:false,”c_isbrandstory”:false,”c_isbrandcontentorbrandstory”:false,”c_isbranded”:false,”c_ishvg360article”:true,”c_partnername”:null,”c_partnerlogo”:”00000000-0000-0000-0000-000000000000″,”c_partnertag”:null},{“available”:true,”c_guid”:”18ff0ff2-8303-467c-afb4-2ff121412d7a”,”c_author”:”hvg.hu”,”category”:”cegauto”,”description”:”A rendőrség a 38-as úton traffipaxozott.”,”shortLead”:”A rendőrség a 38-as úton traffipaxozott.”,”id”:”20201003_50es_szakaszon_134gyel_szaguldott_az_autos_akinek_a_kocsija_sem_volt_rendben”,”image”:”https://img1.hvg.hu/image.aspx?id=18ff0ff2-8303-467c-afb4-2ff121412d7a&view=ffdb5e3a-e632-4abc-b367-3d9b3bb5573b”,”index”:0,”item”:”a1086eaa-ccd4-4ccd-84d2-d93546ac20c2″,”keywords”:null,”link”:”/cegauto/20201003_50es_szakaszon_134gyel_szaguldott_az_autos_akinek_a_kocsija_sem_volt_rendben”,”timestamp”:”2020. október. 03. 07:43″,”title”:”50-es szakaszon 134-gyel száguldott az autós, akinek a kocsija sem volt rendben”,”trackingCode”:”RELATED”,”c_isbrandchannel”:false,”c_isbrandcontent”:false,”c_isbrandstory”:false,”c_isbrandcontentorbrandstory”:false,”c_isbranded”:false,”c_ishvg360article”:false,”c_partnername”:null,”c_partnerlogo”:”00000000-0000-0000-0000-000000000000″,”c_partnertag”:null}]
The number of independent publishing offices of power is steadily declining, and those that still exist are trying to stay afloat with a growing headwind. At HVG we persevere, we do not give in to pressure and we bring national and international news every day.
That is why we ask you, our readers, to support us, support us, join our membership and renew it!
And we promise to keep doing our best for you in all circumstances!

hvg.hu
Technology
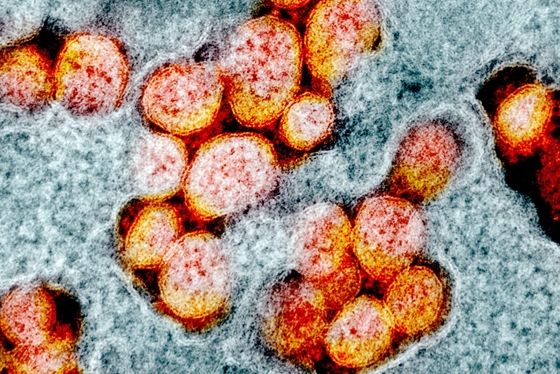
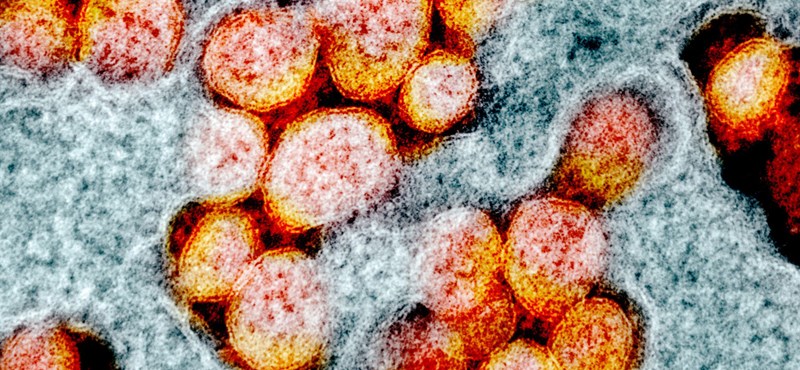
The drugs called Avigan and Favipiravir were developed in 2014 by a Japanese company known primarily for its cameras and photographic supplies. Now it has been found that one of the ingredients in the formulations is also good against coronavirus.

MTI / hvg.hu
Technology
The active substance favipiravir has already been used successfully in Asia in people with coronavirus infections. Its production is also supported by the Hungarian state.
Recommended from the cover

Ivan Stoychev
Economy
Ambassador László Odrobina from Madrid was acquitted by János Áder.
The president of the coronavirus was taken to the hospital as a precaution.
Patients with moderate conditions recover faster from the drug.
[ad_2]


