[ad_1]
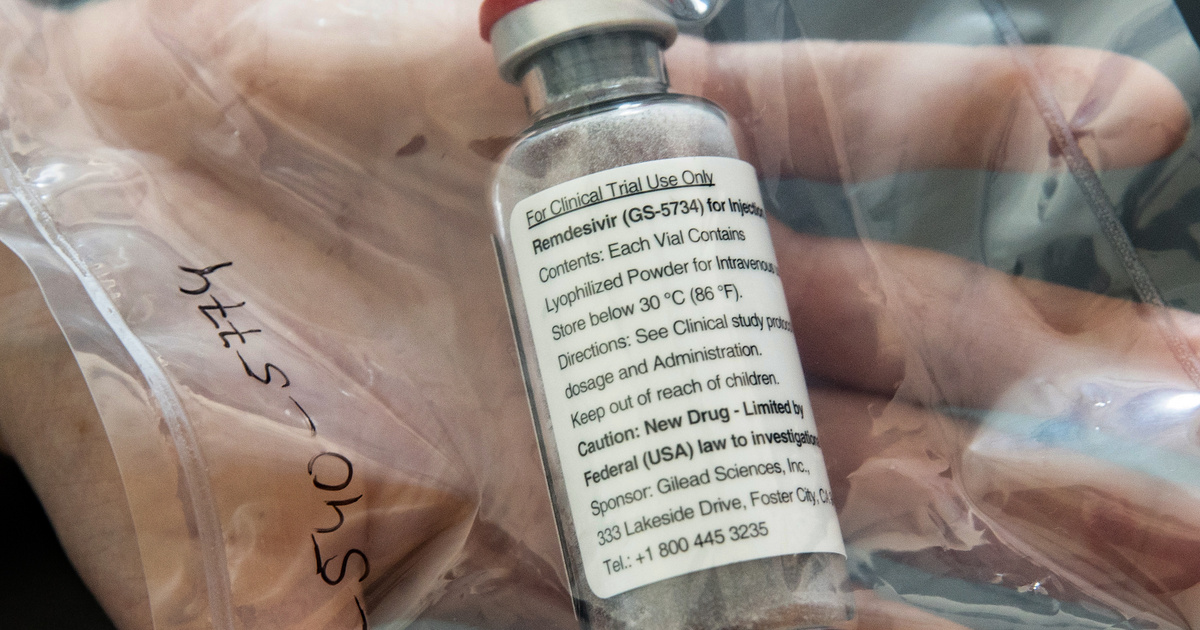
The United States Food and Drug Administration (FDA) has issued an emergency license for an ebole medication called remdesivir, writes CNN. Drug maker Gilead Sciences said a week ago that the drug had proven ineffective, but the FDA is now allowing serious Covid-19 patients to receive the drug intravenously in hospitals.
The decision is based on a study published Wednesday that the drug shortened the course of the disease in seriously ill patients. This license is only temporary and limited, it is not a full FDA license. This means that remdesivir can be used in justified cases, although not in all patients.
The FDA only allows the drug to be used in children and adults who have a suspected or proven infection and who are particularly serious.
The Gilead CEO announced that there is enough remdesivir available for at least 140,000 treatments.
(Recommended image: Ulrich Perrey / AFP)
records
[ad_2]
