[ad_1]
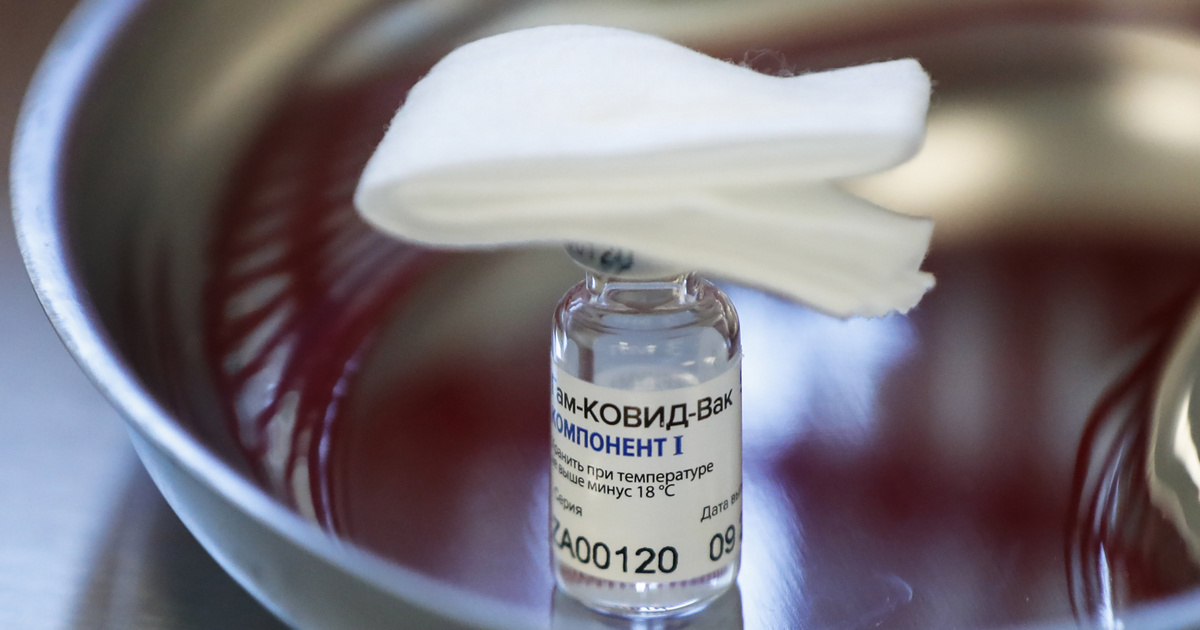
In Russia, two million doses of the Sputnik V vaccine are ready to be inoculated with the population. Under the leadership of Russian President Vladimir Putin, a vaccination campaign will begin this week. Doctors and teachers will receive the first round of the vaccine, which is still only in the third phase of testing, so it is not an officially registered coronavirus vaccine. In the phase 3 clinical trial phase, 25,000 volunteers were vaccinated in Russia. Meanwhile, Miklós Kásler, Minister of Human Resources, announced this Monday
Hungary is considering joining the clinical trial.
The announcement creates a completely new situation and it came unprecedented.
So far, we have learned that the Gamleja National Center, which developed Sputnik, has carried out Phase 1 and Phase 2 studies; We have written that the Russians have received serious scientific criticism on this matter. During these phases, the clinical, pharmacological, and pharmacodynamic effects of the drug are explored. Translated into Hungarian:
They look at the most effective dose, how the drug is distributed in the body and excreted, and side effects are followed. They also look at what therapeutic effects it has and how safe it is.
In Phase 3, they try to validate the results of Phase 2 in a larger group and compare the efficacy and safety with the best similar agent on the market. This is the biggest problem at the moment, as there is no proven vaccine against SARS-CoV-2 type coronavirus that has been used anywhere in the world. Therefore, the developed vaccine can only be compared to a placebo, an ineffective drug.
When the Phase 3 study is completed, its results must be published in a medical journal, that is, presented to the scientific world so that everyone can access the information. This can only be followed by the initiation of the authorization process, the placing on the market of the vaccine in possession of the authorization, and then its delivery in bulk to doctors’ offices. The efficacy, side effects and safety of the vaccine are tested during a long “follow-up” phase, this phase 4.
It can also be administered in a clinical trial.
The Sputnik vaccine, as described above, is in phase 3, which means that people in Russia are now being vaccinated without an official permit. This is unprecedented. In Hungary, for example, a coronavirus drug called remdesivir was initially administered to severely ill patients in a clinical trial.
Therefore, this is not a risky experiment, as the efficacy has already been tested in Phase 1, Phase 2 of the human study, safety in specific patient groups, Phase 3 and Phase 4 can simply make its use be even more secure.
Miklós Kásler’s announcement stirred the stagnant water to the point that until now there has been no talk of Hungarians entering the clinical trial. The Index only received information that the Hungarian drug licensing authority, OGYÉI, was analyzing a document of thousands of pages it had received on the results of clinical trials of the Russian vaccine so far. As far as we know, if this metameta has been analyzed, in case of a positive evaluation, permission can be granted to the Hungarian population to receive the Sputnik vaccine (in the Phase 3 clinical trial phase). (Originally intended for marketing in the European Union by the European Medicines Agency [EMA] can issue a permit, but the authority of a Member State can also act in an emergency according to a 2001 European Commission regulation).
We know that a thorough review of the documents can take months at OGYÉI, so Cecília Müller speaks repeatedly about the daily briefing of the operational personnel who
As long as there is no permit, it is not worth talking about when vaccines can start, as we are still quite a long way from that. On Tuesday he spoke about the first quarter of 2021.
How can Hungary investigate?
If Hungary decides to participate in the Sputnik clinical trial, an official permit is also required, which must also be issued by OGYÉI. Once you have that, you can start to involve 3-5 thousand people. We sent questions to OGYÉI about how people could apply for the study, what doses they would receive, and how long they would test its effectiveness. We await your responses. We also asked them why we wanted to get into the investigation, which has been going on in Russia for a long time. Until we get an answer to this, we can only guess.
There is a hypothesis that it would paint a favorable image of the government if it were soon vaccinated in Hungary with a coronavirus vaccine, even if it is not a licensed vaccine but a clinical trial. In the UK, the vaccination campaign with the Pfizer vaccine will start this week, while Slovenes announced on Saturday that 50,000 people will also receive the Pfizer / BioNTech vaccine in the first round in early January. So the vaccine will be available next door and eventually it seems that
Vaccine competition also has a political dimension: where the vaccine arrives first, the government can build political capital out of it.
The national medical director said in a statement from the operating court on Tuesday that the government was trying to obtain vaccines on various lines, having so far linked 17 million doses of vaccine. On November 19, Gergely Gulyás spoke of only 14.9 million linked vaccines (we had vaccinated them at AstraZeneca, Janssen and Pfizer), according to which the government has been moving forward since then. Great efforts are needed as the SARS-CoV-2 coronavirus vaccine is currently the best-selling vaccine in the world. Pfizer, for example, was found to have canceled a portion of the portion that we had promised them, and the advance was also returned to the Hungarian government, given or given to someone else for business or other purposes.
Although the WHO has established a global mechanism called Covax (Covid-19 Vaccines Global Access) for a fair global distribution of the vaccine, manufacturers are trying to grab as much of the vaccine market pie as possible in their own interest.
We also asked operational staff and the Ministry of Foreign Affairs, which is active in acquiring Sputnik, how much, from which manufacturers, they ordered and ordered the vaccine, and when they could get here, because that could get us closer to when the campaign of vaccination. Cecília Müller only answered partially to this on Tuesday, but if we get the answers to the other half of the questions, we will let you know immediately.
Optional or not?
Cecília Müller also made a statement on Tuesday that they will begin to administer a greater number of vaccines that the Hungarian authorities find safe, so
you cannot choose the type and source of the vaccine.
They can offer what is licensed. He responded to a question from reporters about whether, if multiple vaccines were available, the public could choose which one to order.
The clear answer to this question is also important because Viktor Orbán said in another radio interview in October that it could easily be that up to two or three types of vaccines would be available in Hungary in the spring, and people would be able to choose which one they want to administer.
On Tuesday, the Coronavir Virus Press Center sent a statement to the MTI accusing 444.hu of spreading false news. In an article on the portal, he pointed out that Müller Cecília’s statement could be interpreted as that it will not be possible to choose between vaccines, whatever is available will be injected as long as the Prime Minister has not spoken before.
Cecília Müller was certainly ambiguous, since she stated categorically that it was not possible to choose the type of vaccine, people would receive in large quantities what the Hungarian authority allowed. The point is in the supplement sent to the press center by MTI, and the unequivocal explanation would have been more fortunate if it had also been expressed in the information of the Operational Staff:
“We hope that next spring we will be there to make sure that all foreign vaccines requested by Hungary are available and that there will be a number of vaccines approved by the authority in large numbers, from which we can even choose.”
[ad_2]