[ad_1]
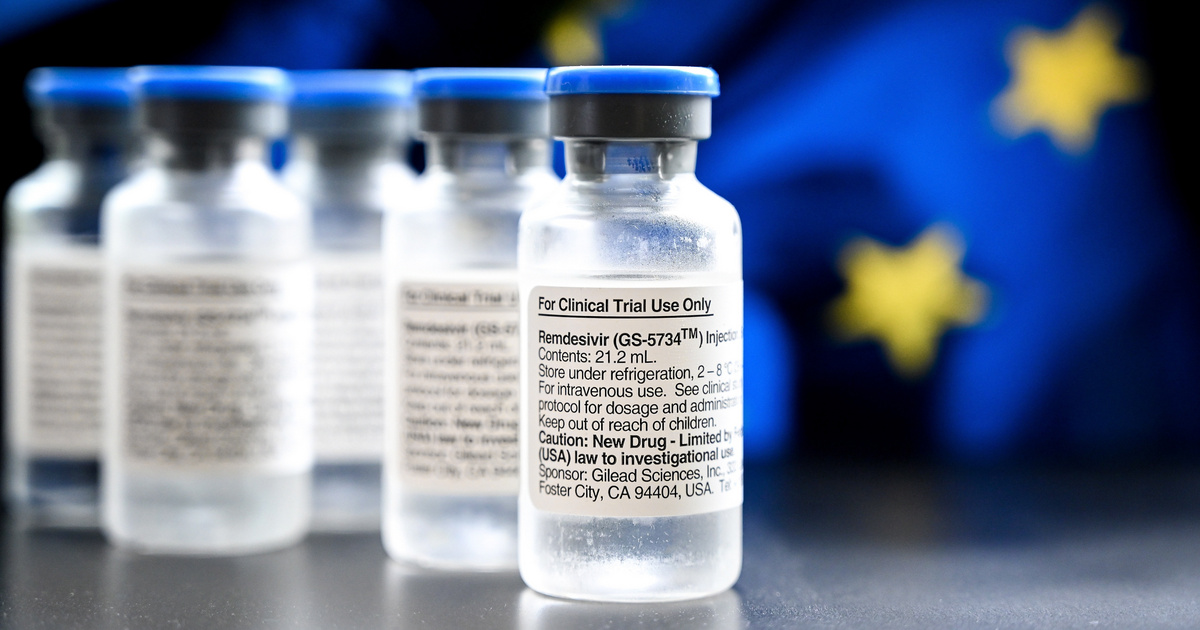
Already 540 people nationwide have been treated with remdesivir, made by the Richter pharmaceutical company, which to date has shown no serious side effects in subjects. The drug is administered to patients in a clinical trial.
As leader of the Hecrin Consortium, which brings together universities and large hospitals, Gábor L. Kovács oversees the network responsible for the Hungarian clinical research infrastructure. In early November, we spoke with him about expanding the range of hospitals involved in remdesivir therapy from seven to fifteen. In comparison, there was a big change:
There was a new direction, a great opening
The professor told Index. Gábor L. Kovács explained that
remdesivir use in a clinical trial stopped to enroll new patients,
which has two reasons.
- On the one hand, start the statistical processing of the results so far.
- On the other hand, the Operational Team ordered remdesivir to be administered to patients not only in a few selected hospitals, but in all institutions authorized to care for Covid patients with the permission of the Minister of Health.
This means that 78 hospitals will receive remdesivir today
Said the professor.
As the range of hospitals began to expand from seven to 15, operational staff came under heavy pressure from institutions that had a Covid department but remdesivir was not available. So they decided to widen the circle. The legal framework for this has also been established, since the National Institute of Pharmacy and Food Science (OGYÉI) has authorized the use of remdesivir before the so-called indication (in international language, “compassionate use”) at the request of the Ministry of Innovation and Technology. This means that prior informed consent from the patient is still required, patient symptoms must be documented, and any side effects of the study drug must be reported, but this type of paperwork is negligible compared to actual clinical trials.
The state enters
The use of remdesivir in a clinical trial has so far been supervised by the HECRIN Consortium, but the pre-indication use is controlled by the Hungarian state due to the nature of the issue.
The state has taken over Richter’s entire stock of investigational preparations and is distributing them to 78 national hospitals through the drug wholesale company (Hungaropharma).
“The medical community is very satisfied with this development, patients will also have much broader access to remdesivir,” said Gábor L. Kovács, a member of the Hungarian Academy of Sciences, but added that it is now one of the hot topics.
that the enormous demand for remdesivir from the 78 institutions will be met?
Richter will produce thousands of blisters in December. (We ask the pharmaceutical company how many batches it can produce exactly, and as soon as we get a reply, we will update our article.) However, it is expected to be ready by the end of February with another dose.
Between December and February, the Hecrin Consortium said the state would have to buy enough drugs from the original manufacturer of remdesivir, Gilead, with no other sources.
Going back to the 540 previous patients who had already received remdesivir: it is still encouraging that they did not experience serious side effects from the doctors, which should have led to the discontinuation of the clinical trial. A follow-up study will be performed 30 days after the last treatment of the last selected patient, and the results can be processed statistically. With this in mind, detailed results on the clinical safety of the Hungarian remdesivir product are expected towards the end of the year.
However, even without detailed processing, we can see that there is certainly no big problem
Gábor L. Kovács closed his words.
[ad_2]