[ad_1]
It is somewhat incomprehensible that the government has not previously released the very good news that Richter has succeeded in synthesizing Remdesivir, which is currently the most effective drug for the treatment of patients with severe COVID, the fruit of five months of hard work. done.
It’s not really a stadium opening, it’s not a transfer, but still!
Richter’s great performance was first reported by the index, just a week ago, last Wednesday. The next day, on M1, Béla Merkely, Rector of Semmelweis University, was asked what she said about the “Richter announcement.” “Injections containing the active ingredient remdesivir are already available in hospital care,” read Hirado.hu, but at the time OGYÉI did not even receive Richter’s request, the clinical trial was only approved on Monday morning, for which presumably the rector did not. I was thinking of the Hungarian factory medicine.
Now, on Monday, when asked by a journalist in a statement from the operational staff, “contrary to rumors, remdesivir is available from its own national manufacturer, thanks to the pharmaceutical company Richter.”
Isn’t Richter earning more?
The volume produced will allow the treatment of about 3,000 hospital patients with remdesivir, the first drug to date that has been clinically shown to speed patient recovery and reduce mortality rates in a similar fashion to a trend, Richter told Index on Wednesday. past.
That doesn’t seem like a high figure when there are more than a thousand new infections a day, there are currently more than 1,500 people hospitalized with a coronavirus – every 10th patient on a ventilator – and dozens of deaths are reported daily on the official site.
On Tuesday evening, we also asked the operational staff and Richter if the Hungarian pharmaceutical company had received another order from the state or if only this amount was produced for 3,000 patients. The operational staff is waiting for inquiries until 9 am, however, none of our questions were read at Wednesday’s press conference, just as Richter did not respond to our inquiry.
However, from the responses of the pharmaceutical company last week, it seems so true that
the government contacted them in March, and Richter produced enough drugs to treat 3,000 patients and delivered them to the state.
No one communicated out loud that production would be continuous – or perhaps as much as ITM said in a statement last Friday that
the prepackaged volume currently available in the manufacturer’s warehouse allows the treatment of approximately 250 patients. By the end of October, a total of more than 800 patients will have access to enough medicines.
In other words, many of the 3,000 have passed through quality control.
400 million for drugs and 300 billion for ventilators
The Ministry also revealed that the company would deliver the drugs manufactured as a result of the government-funded development to the Hungarian state.
the project can be implemented with a grant of some 400 million guilders.
Here’s a very serious number: 400 million guilders. What does not seem to be a significant amount in the fight against the epidemic at all, it is enough to think only of the 16,000 fans for which the state spent HUF 300 billion, and their storage alone is a billion item. The other problem is that there are not enough nurses who can handle them.
And a drug that looks promising, according to current government communications, looks a lot like “just” enough to treat 3,000 patients.
However, of course, it is conceivable that the government, in the meantime, has also placed orders from other manufacturers, and that the drug is available somewhere. In May, the news was that Hungary was among the first to participate with Poland in the trial of intravenous remdesivir, and it was said that clinical trials would begin in early May at St. László Hospital. It is a Gilead Sciences drug that was originally developed against Ebola.
Richter’s drug can already be used in seven institutions
The institutions also received the necessary permits for the clinical trial of the drug containing the active ingredient remdesivir, so that on Monday the first patients could have access to the drug manufactured by Richter, according to Csaba Index Lengyel, president of the Albert Szentgyörgyi Clinical Center of the Szeged University.
Csaba Lengyel told the Index
For this drug to be widely used in Hungary, it must go through several phases of clinical research.
The research phase of Phase 3 examines the safety, tolerability, and efficacy of the drug.
The study, that a hope It was named – organized in record time – more than 30 people worked during these three days, day and night. This required the very close cooperation of several participants: Csaba Lengyel highlighted the background institutions of ITM and EMMI, the logistical cooperation of operational staff and the Ethics Committee of Clinical Pharmacology of OGYÉI and ETT. As a result of five months of hard work, Richter successfully solved and synthesized the active ingredient remdesivir, which is currently proving to be the most effective in treating patients with severe Covid disease.
Reaching that level in five months is not normal, as it is not
The professor declared.
The drug itself is used in other parts of the world, and the really important meaning of the study released Monday is that it will be widely available to Hungarian patients.
It is the most effective drug known to date against Covid infection, and Hungary could become self-sufficient thanks to Richter’s pharmaceutical plant and the study that is now underway.
According to Csaba Lengyel, it was a very big and hard job that he managed to get permission from OGYÉI on Monday, so that the investigations can begin. The main coordinator of the research is the Hungarian Clinical Research Infrastructure Network (HECRIN), headed by academic Gábor Kovács. It is a body created to support academic research that coordinates such studies, such as the clinical trials against Covid in Hungary. The professor stressed that
the doctors taking part in the exam do not receive any financial compensation, the various tasks are performed by everyone in their work,
and pharmacological tests are often financially rewarded; now, given the situation, no one has requested one.
Where is it used?
On the side of patient care, the university, which includes the four schools of general medicine in the study,
- the University of Szeged,
- Semmelweis University,
- the University of Debrecen and
- the University of Pécs, and
- South Pest Central Hospital,
- the National Institute of Early Pulmonology and
- the New St. John Hospital
participate in clinical research.
The drug will already be used in these institutions.
“Patients infected with Covid who have reached the age of 12 and are in the intermediate stage of infection can receive the drug,” said Csaba Lengyel. He said the drug is an intravenous infusion that has a starting dose followed by a maintenance dose. Five to ten days is a course of medication followed by a 30-day follow-up as part of a clinical trial.
The professor highlighted that this drug has proven extremely effective against Covid infection:
According to international research, it can reduce the risk of death by up to 50 percent, which is a very large number.
Who can get it?
You cannot request this test separately. Those who are admitted to these institutions and are eligible to receive this drug will begin treatment in patients after consent has been given. It will be used in patients who
- You have a PCR-confirmed Covid infection or clear symptoms even without a PCR test (for example, based on a chest CT scan), or
- the patient already needs additional oxygen therapy.
“This is a single-arm, open-label study: everyone gets the drug, everyone gets an active ingredient, there is no control group,” said Csaba Lengyel, adding that the study continually collects basic data on efficacy. and drug safety.
The product will not be marketed to the general public,
Being a parenteral drug, that is, the active ingredient is injected into the body, so it cannot be marketed, it is only prepared for hospital use.
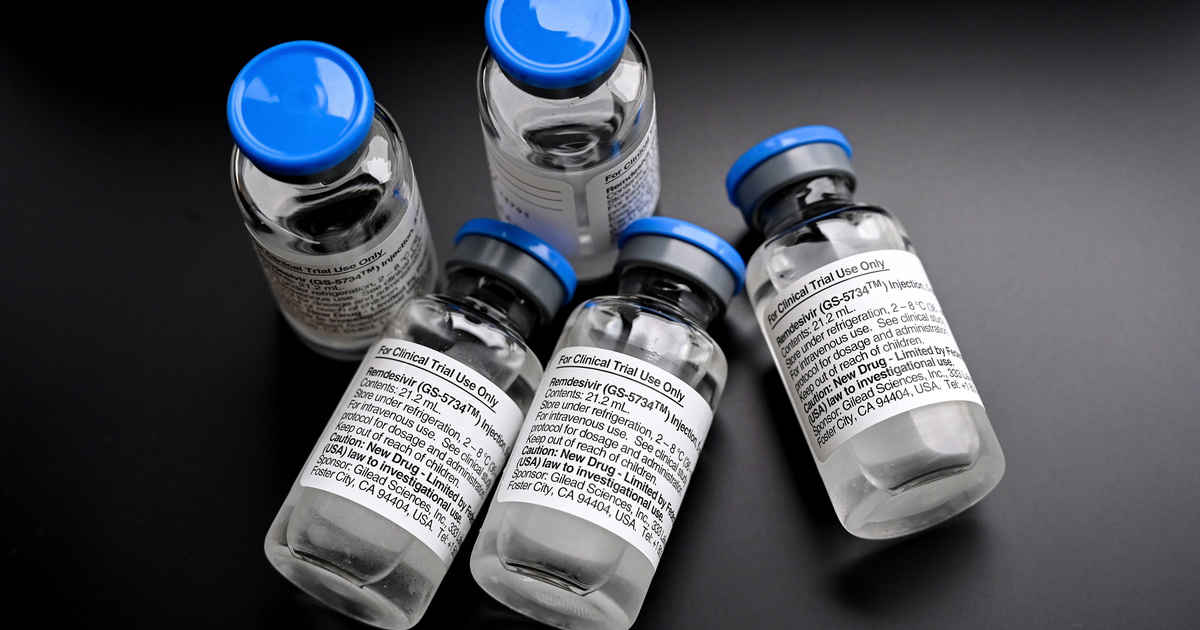
(Cover image: Ampoules filled with the antiviral Remdesivir at Essen University Hospital on June 3, 2020. Photo: Sascha Steinbach / MTI / EPA)
[ad_2]