[ad_1]
In a recent study, researchers showed that
Stem cells derived from stem cells can integrate well into appropriate brain regions, connect with native neurons, and restore motor functions.
Decreased nervous system
A Parkinson’s disease a disease of unknown origin that involves the destruction of areas of the brain that work with a neurotransmitter called dopamine. By the time symptoms occur, patients have already lost at least eighty percent of the affected brain cells and the connections between them – the synapses. The classic symptoms are usually immobility, rapid fatigue, hand tremors, postural instability, slowed thinking and dementia. Most patients do not develop all of the listed symptoms at once. It is important to note that there are diseases that cause similar symptoms but they are not Parkinson’s. In Wilson’s disease, for example, the body lacks a specific transport protein, ceruloplasmin, which causes copper deposits in the liver and central nervous system. The disease presents with similar symptoms.
Parkinson’s disease remains an incurable, progressive and chronic disease. Its formation is unknown, however, there are potential factors: attack from certain infections, physical trauma, stroke, and free radicals. Its pharmaceutical forms of treatment are aimed at replacing the decreasing amount of dopamine in the brain, promoting its effect and inhibiting substances that have an anti-dopamine effect in the body. In the case of surgery and electrical intervention, malfunctioning areas of the brain are put out of commission, reducing the number of confusing and dangerous situations. However, they are only suitable for treating and preserving the patient’s condition, they cannot cure problems.
Stem cells in the target cross
That is why the staff at the University of Madison-Wisconsin began experimenting with stem cells after several proven ideas. Stem cells are unspecialized cells that can assume a specific function (pluri or multipotent) under the influence of certain biochemical signals and, therefore, can be activated in the regenerative healing process. Scientists have discovered in a mouse model that stem cell-derived neurons can integrate well into appropriate regions of the brain, connect with native neurons, and restore motor and motor functions.
The most difficult task for the research team was “developing” the stem cell used in the mouse experiment to make the already damaged dopamine-producing neurons in the brain and their synaptic connections.
Su-Chun Zhang, head of the researchers involved in the study, on September 22, published the results of the Cellular stem cell in a diary. The research was assisted by Yuejun Chen, Man Xiong and Yezheng Tao, postdoctoral researchers from the Zhang Laboratory, who now hold positions at university faculties in China and Singapore.
The correct stem cell neuron is the most important factor, as it is able to enter damaged neural connections as a healthy partner and give the correct electrical and biochemical impulses, which is why it works.
Says Zhang, a professor of neuroscience at the UW-Madison Waisman Center.
Neurological (nerve) injuries usually affect specific brain regions or specific cell types. The functional loss of these causes damage to neuronal connections and symptoms and consequences. To treat diseases, we need to restore these neural circuits and synapses, then the disease can become curable.
In a mouse model of Parkinson’s disease in an experiment, researchers began to repair circuits damaged by
Human pluripotent embryonic (intrauterine, umbilical cord) stem cells have specialized in dopamine-producing neurons and have adapted to cells that die in disease.
These new neurons were transplanted into the midbrain of mice, one of the brain regions most affected by Parkinson’s degeneration.
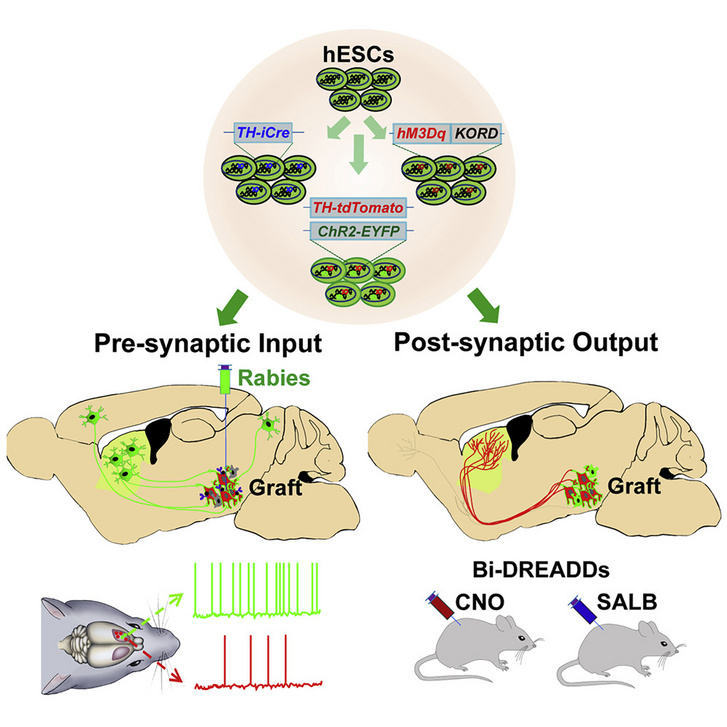
Photo from ars.els-cdn.com
A few months later, the researchers realized that the mice’s movement function had improved. Zhang and his colleagues observed the animals closely and withdrew from the results of the CT scans: the neurons of the mice grew to multiple sizes to reach groups of intact movement-regulating neurons. However, it is important to note that only the dopamine-producing cells showed such a special change: the stem cells did not start growing into other neurons that produce different neurotransmitters,
which shows that stem cells must be specifically developed to fit into the appropriate synaptic circuitry rather than the damaged neuron.
Researchers have shown that the effectiveness of stem cell implantation: Activating and deactivating genetic elements have been inserted into stem cells.. When it was turned off, the motor functions and neural connections of the developing mice disappeared, suggesting that the stem cells are essential for restoring the Parkinson’s damaged brain area. And turning the genetic switch back on has resulted in fantastic progress.
Genetic change technology has also been shown to be suitable for adjusting the activity of transplanted cells to optimize and personalize treatment.
For years, Zhang’s research team has been developing methods to convert stem cells into different neurons in the brain.
Each neurological disease or injury requires its own special neurons, but the treatment plans would likely be more or less similar. Parkinson’s disease was used as a model, but the principle is the same for a wide variety of neurological disorders.
Zhang says.
(Cover image: MRI of a patient’s brain during a deep brain stimulation operation in the operating room of the National Institute of Neuroscience in Budapest on December 15, 2012. MTI photo: László Beliczay)
[ad_2]