[ad_1]
The drug was developed by University College London Hospitals and"AstraZeneca", The pharmaceutical company that has developed in association with the University of Oxford, an anti-Corona vaccine, which is expected to be approved by the health authorities of Great Britain next week.
The team hopes the experiment will show that the mixture of antibodies in the drug protects against coronavirus infection for a period of 6 to 12 months.
Trial participants receive two doses and, if approved, will be offered to a person exposed to"COVID-19" In the previous eight days.
The new drug may be available in March or April, if approved by UK pharmaceutical industry regulators, after reviewing the results of the studies.
The University of California, Los Angeles and several British hospitals are participating in the trials, as well as a network of 100 locations around the world.
Catherine Hollihan says: "So far, we have injected 10 participants who were exposed to the virus at home, in healthcare settings or in student residences."And your co-workers will closely follow the participants to see which one develops"COVID-19".
She adds: "The advantage of this drug is that it provides you with instant antibodies. We can tell trial participants who have been exposed to the virus: Yes, they can get vaccinated. But we will not tell you what will protect you from the disease, because it is too late (since my vaccine is "Pfizer Bionic" AND"Oxford" They don’t achieve full immunity for about a month after receiving one of them)".
According to Paul Hunter, a professor of medicine at the University of East Anglia, Great Britain, who specializes in infectious diseases, the new treatment can significantly reduce the number of deaths from the Corona virus.
Hunter says: "If you are facing outbreaks in places like nursing homes, or if you have patients who are at particular risk of contracting the coronavirus, such as the elderly, this could save many lives. If effective in phase III trials, it can play an important role in the preservation of humans.".
“>
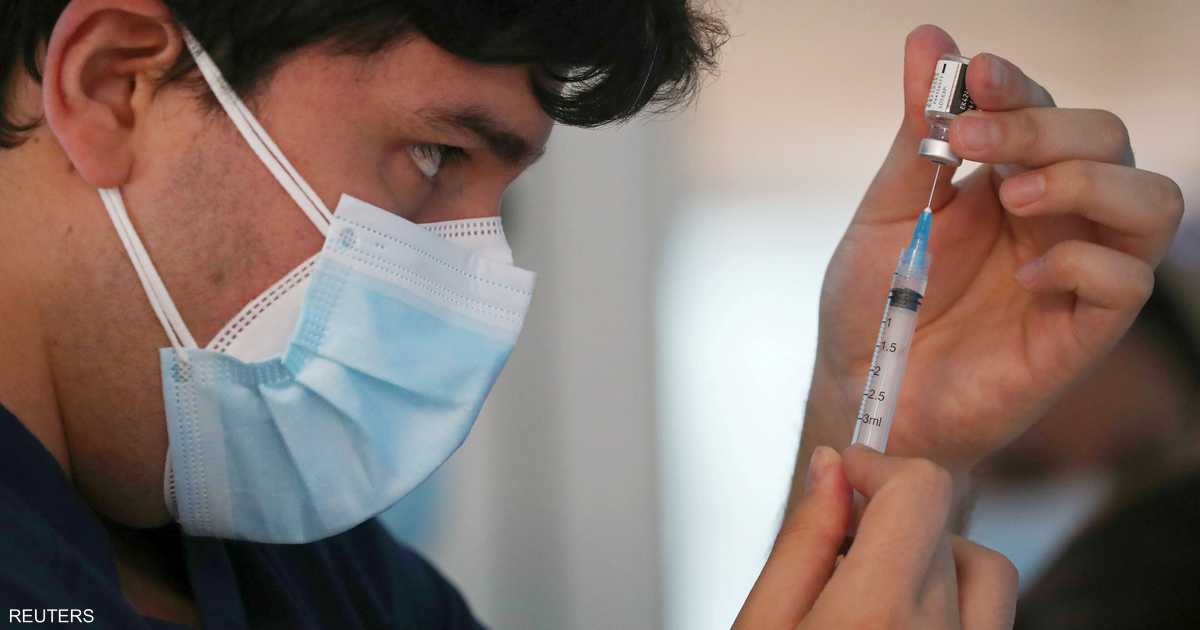
The drug contains a group of long-acting antibodies that were prepared in the laboratory, developed by the British company “AstraZeneca” for the manufacture of drugs, and experts say that the drug gives humans antibodies against the Corona virus “immediately” .
According to the study highlighted by the British newspaper “The Guardian”, the proposed antibody treatment gives immediate immunity to the disease, and can be administered as emergency treatment to hospital patients and residents of nursing homes, to help contain the outbreak of the epidemic that has terrorized the world.
The drug can also be used with people living in households where someone has been infected with the emerging coronavirus, to ensure they are not exposed to the infection, and it can also be administered to college students, among whom the virus spreads rapidly. , because they live, study and communicate together.
Catherine Houlihan, a virologist at University College London Hospitals, who is leading a study on the drug, says: “If we can show that this treatment works and prevents people exposed to the virus from continuing to develop (Covid 19), then it will be an exciting addition to the weapons that are being produced to combat this. The horrible virus. “
The drug was developed by University College London Hospitals and “AstraZeneca”, the pharmaceutical company that, in partnership with the University of Oxford, manufactured an anti-Corona vaccine, which is expected to be approved by the UK health authorities next week. .
The team hopes that the experiment will show that the mixture of antibodies in the drug protects against infection with the coronavirus for a period of 6 to 12 months.
Trial participants receive two doses and, if approved, will be presented to a person exposed to “Covid 19” in the previous eight days.
The new drug may be available in March or April, if approved by UK pharmaceutical industry regulators, after reviewing the results of the studies.
The University of California, Los Angeles and several British hospitals are participating in the trials, as well as a network of 100 locations around the world.
“So far, we have injected 10 participants who have been exposed to the virus at home, in healthcare settings or in student residences,” says Catherine Houlihan, and she and her colleagues will closely follow the participants to see which of them will be infected with “Covid 19”.
She adds: “The advantage of this drug is that it provides immediate antibodies. We can tell trial participants who have been exposed to the virus: Yes, they can get the vaccine. But we will not tell them what will protect them from the disease, because it is already too late (since my shots are Pfizer “). Bionnnet and Oxford do not grant full immunity until about a month after receiving one of them.)
According to Paul Hunter, a professor of medicine at the University of East Anglia in Britain, who specializes in infectious diseases, the new treatment can significantly reduce the number of deaths from the Corona virus.
“If you are dealing with outbreaks in places like nursing homes, or if you have patients who are particularly at risk for coronavirus, such as the elderly, this could save a lot of lives,” Hunter says. If it is effective in phase III trials. It can play an important role in the preservation of human beings. “
[ad_2]