[ad_1]
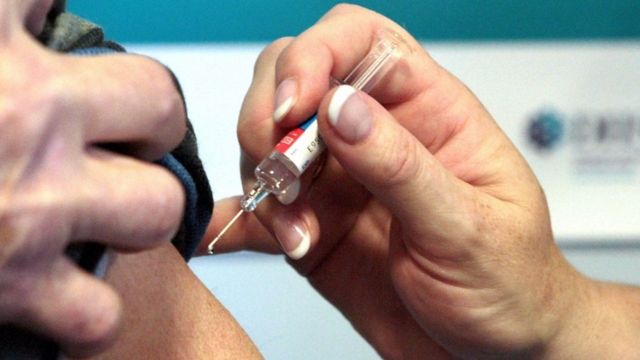
Image posted, David Cheskin / PA Wire
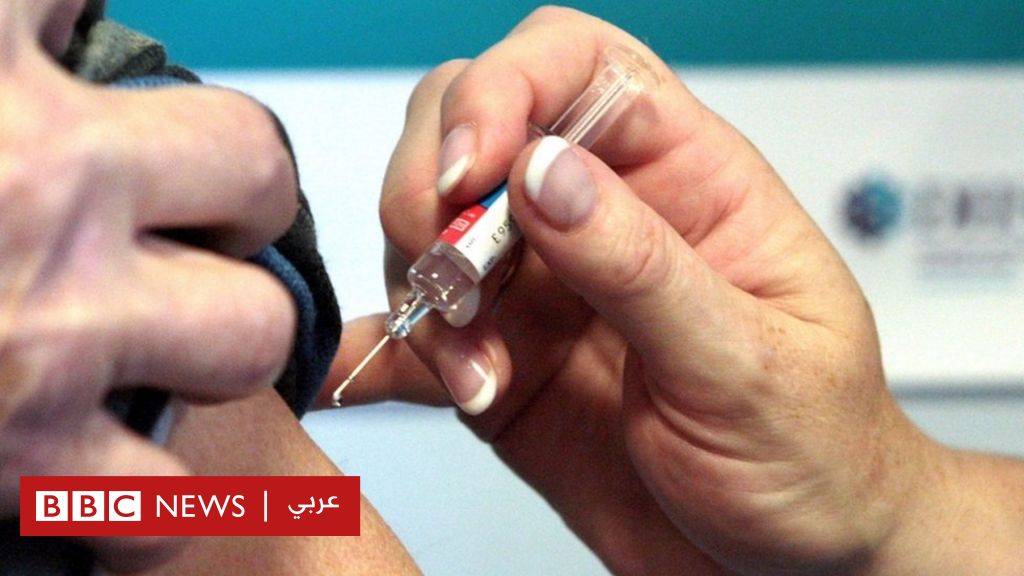
The trial of a possible vaccine against coronavirus, developed by the University of Oxford and AstraZeneca, showed a strong immune response in adults in the sixth or seventh decade of life.
This rekindles hopes that the vaccine can protect members of these age groups who are most at risk of contracting the virus.
The researchers say the results of the second phase of the vaccine trial, involving 560 healthy adult volunteers, were “encouraging.”
They add that they are also testing whether the vaccine can stop the occurrence of Covid-19 in people, as part of the third phase trials.
First results are expected at this critical stage in the coming weeks.
A volunteer who participated in the Oxford trials.
The companies Pfizer, Biontec, Sputnik and Moderna announced good initial results of three vaccines during the trials of the third phase, one of which reached 94 percent in terms of protection against Covid-19 in people older than 65 years.
Britain has already ordered 100 million doses of the Oxford vaccine, manufactured by the company “AstraZeneca”, 40 million doses of the “Pfizer-Biontic” vaccine and five million doses of the “Moderna” vaccine.
The challenge in developing a Covid vaccine is to stimulate the body to fight the virus, regardless of the age of the person.
But a weak immune system in the elderly means that vaccines do not work as well as in younger people.
Results from Oxford University experiments reviewed by researchers and scientists from the Lancet group indicate that this may not be a problem.
Image posted, fake images
These results showed that older adults, between the ages of 56 and 69 and those older than 70, had a similar response to younger adults, in terms of immunity, between the ages of 18 and 55.
Protects to the maximum At risk
Dr Mahshi Ramasamy, a researcher at the Oxford Vaccine Group, said: “We were happy that the bodies of the elderly accepted our vaccine and responded to it, and it also boosted the immune system to a similar degree in younger volunteers.” .
He added: “The next step will be to find out if this means protection against the disease itself.”
Two weeks after the volunteers took the second dose, the bodies of more than 99 percent of the participants responded by creating antibodies, and this included individuals of all ages.
The T-cell response, another measure of the immune system response, peaked two weeks after the first dose of the vaccine, regardless of age.
Dr. Ramasamy said, “The strong antibodies and T-cell responses seen in the elderly in our study are encouraging.”
He added: “Groups at risk of developing serious illness from Covid-19 include people who already have health problems and the elderly.”
“We hope this means that our vaccine will help protect some of the most vulnerable people in society, but more research is needed to confirm this.”
And during testing, the elderly were also less likely to have side effects, which are generally mild.
There were no serious safety concerns with the vaccine, called “Chadox 1n Cove-2019”.
The volunteers in the experiment were divided into groups and given one or two doses of the vaccine, or the placebo vaccine. Then, assess the reaction of your immune system on the day you received the vaccine and after one week, two weeks, and four weeks after the two doses.
Image posted, fake images
The Oxford vaccine is made from a weakened version of the common cold virus (known as adenovirus) from chimpanzees.
Work on the vaccine began in January and took less than three months to complete. Human trials began, first in Europe, in April at Oxford.
Trials of the third phase of the vaccine, which analyze its efficacy in protecting people from Covid-19, began in late August and are still ongoing.
And when the data from this stage is sent to regulatory authorities, reviewed and approved, the vaccine could be given the green light for use in people around the world.