[ad_1]
Dr. Albert Burla, Pfizer president and CEO, said in a statement Monday about the vaccine’s effectiveness: "Today is a great day for science and humanity. The first set of results from the phase 3 vaccine trial, Covid 19, provides preliminary evidence that our vaccine can prevent corona".
"We have reached this critical milestone in our vaccine development program at a time when the world needs it more than ever, with infection rates setting new records, hospitals close to capacity, and economies struggling to reopen. We are approaching an important step in giving people around the world the achievement they desperately need to help end this global health crisis. We look to share additional safety and effectiveness data generated by thousands of participants in the coming weeks. ".
And so far, there are still unanswered questions about the vaccine’s effectiveness by age and race, and to what extent immunity persists, according to Reuters reports.
“>
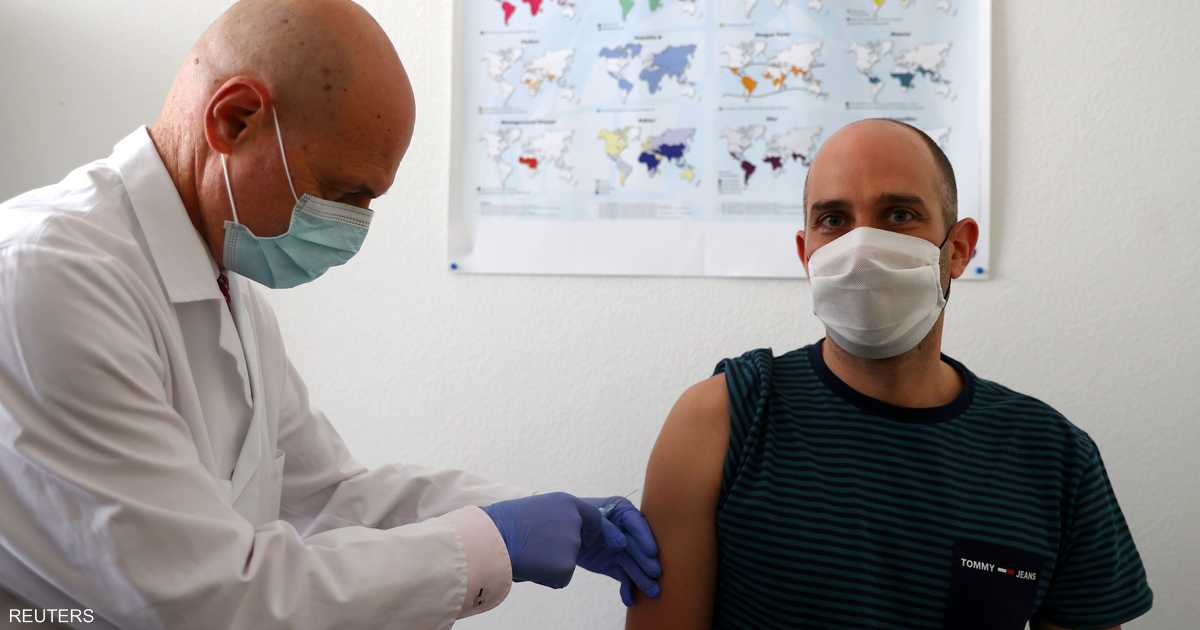
Reports of side effects resulting from the vaccine, which has so far been shown to be safe, come days after Pfizer and its German partner Biontech announced that the vaccine was 90 percent effective in preventing the Corona virus, as it was expects drug companies to apply for a US license to use it in the cases. Emergency”.
During the trials, no more than 43,500 volunteers from six countries were informed whether they had received the vaccine or a placebo.
However, it was reported that some volunteers said they were able to realize they had received the vaccine due to some side effects such as headache and muscle pain.
According to Glenn Deshields, 44, of Austin, Texas, the side effects of the vaccine were similar to “severe hangover headaches,” but they disappeared quickly.
An antibody test that Deschilds later did showed that he had developed antibodies to the virus, which convinced him that he had received the actual vaccine.
Another volunteer, a 45-year-old Missouri woman known as Carrie, said she had suffered a fever, headache and body aches after receiving her first injection, which she compared to a flu shot, with side effects similar to those of the flu, but it was more serious when she got her second injection.
Dr. Albert Burla, president and CEO of Pfizer, said in a statement Monday on the efficacy of the vaccine: “Today is a great day for science and humanity. The first set of results from the vaccine trial Phase 3, Covid 19, provides preliminary evidence of our vaccine’s ability to prevent Corona. “
“We have reached this critical milestone in our vaccine development program at a time when the world needs it more than ever, with infection rates setting new records, hospitals on the brink of capacity, and economies struggling to reopen. We are getting closer. a major step towards supplying people. ” Around the world, with a much-needed achievement to help end this global health crisis. We look forward to sharing additional efficacy and safety data generated by thousands of participants in the coming weeks. “
And so far, there are still unanswered questions about the vaccine’s effectiveness by age and race, and to what extent immunity persists, according to Reuters reports.
[ad_2]