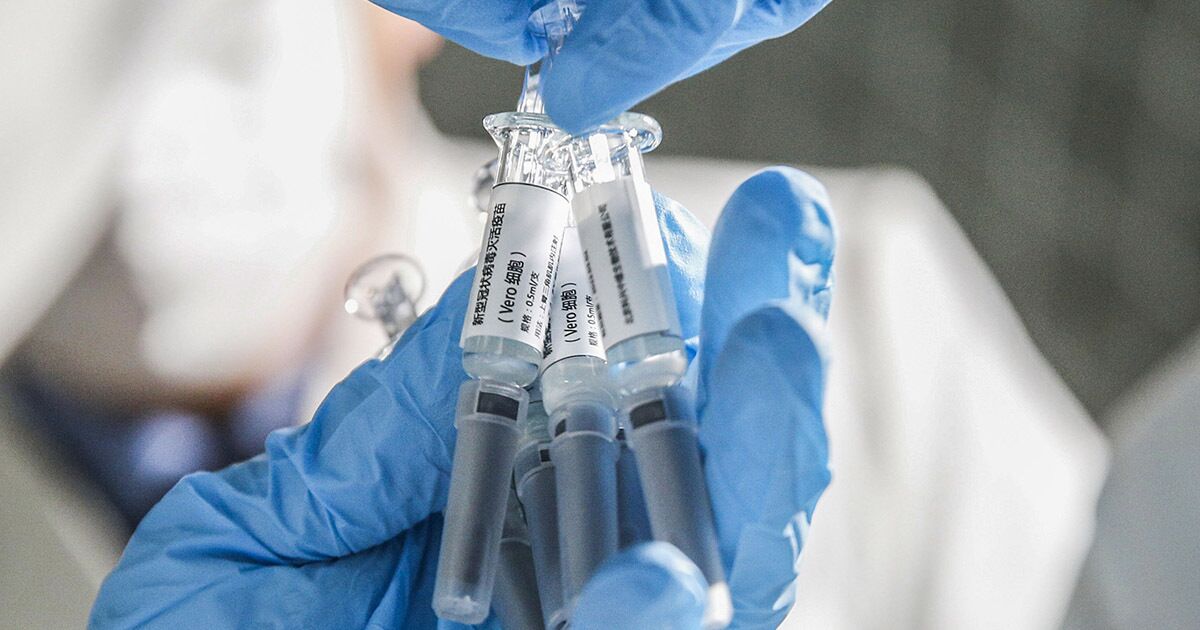

Inactivated Covid-19 vaccine samples at a Sinovac Biotech facility in Beijing on March 16.
Photographer: Xinhua / Zhang Yuwei / Getty Images
Photographer: Xinhua / Zhang Yuwei / Getty Images
Many of the 200-plus Covid-19 vaccine projects around the world are focused on new technologies – inoculations based on, for example, messenger RNA, or genetically modified cool viruses. The company, which is developing one of China’s leading vaccine candidates, is betting that humanity’s best chance may lie with a shot that is not too different from the kind that has been in use for hundreds of years.
Beijing based Sinovac Biotech Ltd., a company with a strong medical track record but a turbulent business history, began final stage tests on CoronaVac in July. It relies on an inactive version of the new coronavirus to teach the human immune system to recognize and destroy the real thing. In terms of timing, the company was ahead of most other potential faxes, including the new models intended to facilitate strong protection and rapid production. The Sinovac candidate has a good chance of entering commercial production almost as soon as possible Modern Inc.’s mRNA vaccine, as the genetically modified shot is developed by the University of Oxford and AstraZeneca Plc.
Sinovac’s method is relatively crude, relying on a similar principle as the one Edward Jenner, the 18th century British scientist sometimes called the father of immunology, used when he used the mild cowpox virus for performing the first impositions against smallpox. But it can just work – and it could be easier to use than the never-before-tried approach of other companies.
“We need to learn about history and not forget what has worked in the past,” said Michael Kinch, a vaccine specialist at Washington University in St. Louis. Louis. “Do not be fancy as simple as it will do just for you.”
There is a case to be made that traditional faxes can be most effective at stopping Covid-19, which has killed about 800,000 people worldwide. Many of the new approaches to faxing focus on replication of the distinction “Spike” protein on the surface of the coronavirus, which helps invade human cells. Data suggest that if the immune system can block that protein, the virus’ attack will be significantly stunted, if not stopped. But it could be preferable to expose the body to as much as the whole virus, giving it a much broader choice of potential targets and letting the immune system decide which ones are most vulnerable.
The benefit of a high-virus approach is that “you use many types of protein,” says William Haseltine, a pioneering AIDS researcher who now chairs Access Health International Inc., a New York-based think tank. “Many of these proteins can induce cell-mediated immunity” – an attack by so-called T cells, for example, instead of by ordinary antibodies. It is far from certain, he says, “but it is clearly a possibility.”
Other benefits may emerge when it comes time to produce and distribute the shot. Although no mRNA vaccine, for example, has ever been licensed for human use, manufacturers have decades of experience with inactivated virus inoculations, making production problems unlikely. And those faxes generally do not need to be frozen, a major injury in developing countries and rural areas where cooling capacity may be limited. (CoronaVac, for example, can be stored at between 36F and 46F.)
On the other hand, inactivated vaccines may need several booster shots to achieve strong immunity, and production means treating large quantities of a dangerous virus. In theory, mRNA vaccines should also be much faster to produce, as they require only small volumes of raw material to produce millions of doses.
Winning approval for CoronaVac would be a huge coup for Sinovac – and for Chinese President Xi Jinping’s attempt to put the country first in delivering a fax. The company is led by Yin Weidong, who began his career in the early 1980s at a public health unit in the northeastern city of Tangshan. On his first day of work, he was assigned to a group investigating hepatitis outbreaks in the surrounding countryside. He went on to spend much of his career concentrating on how to prevent and manage the deadly liver disease in rural China. He founded Sinovac in 2001, not long before a former coronavirus – the one that causes severe acute respiratory syndrome, such as SARS – invaded the country. Sinovac developed a potential SARS vaccine, but the disease disappeared before it could be used. The company later won approval for a shot aimed at H1N1, as swine flu.
When coronavirus infections began to increase in China earlier this year, Sinovac began evaluating its options for developing a new vaccine, Yin says. Everything was on the table, including a high-tech, genetically engineered approach. Business leaders decided that a proven method had the best chance of success in a fast-moving pandemic. “We had the technology ready,” Yin says. “As early as 2003, when we were vaccinated for SARS, we learned a lot about the biology of coronavirus, how to inactivate it and grow it in batches.”
To produce its inactivated virus, Sinovac obtained samples of the Covid-19 pathogen from patients around the world and allowed them to multiply into vero cells, which are derived from the kidneys of monkeys. It then inactivated the virus with beta-propiolactone, a chemical derivative of formaldehyde commonly used in vaccine research, before purifying it into a product suitable for injection – first in mice, rats and primates, and then in humans in early studies. .
The company is awaiting data from its final-stage trials, including a study of about 9,000 people in Brazil that will determine if the vaccine is safe and can prevent disease on a large scale. If the trials are successful, Sinovac plans to produce at least 300 million doses per year in its own factories, while tapping other manufacturers for further production.
However, investors looking for a piece of Sinovac’s potential success will be disappointed. The shares, which are listed on Nasdaq, have been suspended from trading for more than a year, as a result of a complex and unresolved dispute with some of their own investors. The drama began in 2016, when two groups of shareholders – one led by Yin and another by Pan Aihua, Yin’s former mentor and chairman of one of Sinovac’s largest shareholders – made competing offers to keep the company private. to take.
The board eventually approved Yin’s offer, prompting a higher bid from Pan. Two years later, in 2018, a group of investors sought to remove Yin and several other executives from the board. In response, Sinovac moved to massively dilute its shares to diminish the power of Yin’s critics, triggering the trading halt.
The share dispute remains before the courts, and in June Sinovac said it could not estimate when he would be able to resume trading. That means it has recorded nothing like the rising profits of Moderna, whose shares have been driven in value since the beginning of the year amid optimism about its Covid-19 vaccine. Moderna is now worth about $ 27 billion – 60 times Sinovac’s valuation of roughly $ 460 million, which has been frozen since February last year.
All of this is hardly a concern for the Chinese government, as it has identified rapid vaccine development as a key step in its post-pandemic geopolitical strategy – and an opportunity to show that its biotech companies can compete with the best. Beijing regulators are pursuing cheap approvals and allowing experimental immunizations to enter human subjects at an unusual pace – for example, by allowing developers to submit data because they were collected instead of at the end of a research phase. Without those measures, Yin says, Sinovac could never have entered the rapid stage of testing so quickly. Xi also promised share all successful faxes worldwide, which can build goodwill with poorer countries afraid of exclusion by projects such as Operation Warp Speed, the Trump administration’s vaccine program.
Several other Chinese companies are good at human trials, including Tianjin-based CanSino Biologics Inc., which has developed a vaccine that uses a harmless adenovirus to mimic the coronavirus’ spike protein – a similar approach by the Oxford group. Meanwhile, a medical research institute affiliated with the Chinese military is conducting a test for an mRNA vaccine, similar to the one made by Moderna. Another front-runner, state-controlled China National Biotec Group, uses an inactivated virus approach along the same lines as Sinovac’s.
Even if Chinese companies succeed in getting their faxes approved, they will face a formidable challenge: convincing people that their products are safe, especially after a drastically accelerated approval process. Ironically, that can be the most difficult part of their home market, where many consumers prefer Western pharmaceuticals. China’s fax industry has seen repeated scandals in recent decades. A court in Beijing ruled in 2017 that an official at the country’s drug regulator received incorrect payments from vaccine manufacturers including Sinovac. The U.S. Department of Justice and the Securities and Exchange Commission conducted related investigations into the company. Sinovac was never charged in either country.
A broader writing happened about subpar vaccines from some Chinese companies that may have failed to protect beekeepers from diseases such as diphtheria and whooping cough. Last year, Beijing introduced a law for the company’s police, implying further stricter rules on development and threatening heavy fines as a prison term for violations.
Sinovac is envious of demonstrating that it can meet the same standards as American and European competitors. It published the results of his animal experiments in the journal Science in May, reported that his vaccine for coronavirus had produced effective antibodies across multiple species. Data from his early human trials, which the company said showed the injection was safe and produced an immune response, have so far only been provided in a press release, not a peer-reviewed journal, but Yin says the company’s goal is all about making his results available. “We can present every test, every product and every result for worldwide control,” he says. “And we can compare with other products, that it will be clear who is good and who is not.”
If Sinovac’s final phase of tears is successful, CoronaVac could be deployed on a large scale next year. For health experts, each geopolitical consequences of the company ending up at or near the front of the package are far less important than the medical benefits of having more options to protect against the coronavirus. “If we have one vaccine that works, the world will fail,” said Mike Turner, head of major scientific investment at Wellcome, a London-based medical foundation. “We have to have several different faxes. It is not that we have one miracle silver bullet. ”-James Paton, Dong Lyu, and John Lauerman
Next read: Serum Institute of India is ready to produce a vaccine for Coronavirus
.