[ad_1]
The university of oxford begins clinical trials of a coronavirus vaccine on Thursday in the very ambitious hope that it will be available before the end of the year and turn the page on confinement.
Of the more than 100 research projects carried out worldwide to find a vaccine – the only possible way to return to “normal” according to the United Nations – eight are currently in the phase of clinical trials, according to the London School of Hygiene and Tropical Medicine.
These types of tests have already started in China and the United States and are expected to start at the end of the month in Germany, where the federal authority in charge of vaccines gave the green light on Wednesday.
The Oxford University project has strong backing from the British government – it was Health Minister Matt Hancock who announced the start of human testing.
Addressing a House of Commons gathered largely by videoconference, Hancock hailed a “promising development” on Wednesday that would normally take “years” to reach this phase of the investigation.
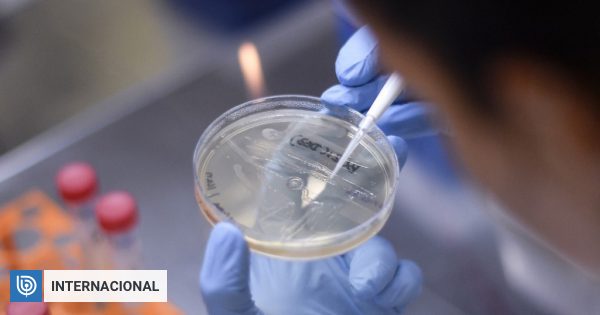
In its first clinical stage, the trial conducted by the Jenner Institute at the University of Oxford to evaluate the safety and efficacy of the vaccine will involve 1,112 volunteers: 551 will receive one dose of the potential covid-19 vaccine and the same will serve as control group when unknowingly receiving an injection without active ingredient.
The remaining ten participants will receive two doses of the experimental vaccine, at a four-week interval.
Professor Sarah Gilbert’s team estimates its success probabilities at 80% and in parallel with the research, it plans to produce a million doses until September, to have wide availability before the end of the year if its efficacy is confirmed.
It is an “extremely ambitious” schedule and could change, the researchers acknowledge.
Boris Johnson government chief medical adviser Chris Whitty warned Wednesday that the probability of getting an effective vaccine or treatment for covid-19 “within the next year is incredibly low”. Meanwhile, he warned, “we have to be realistic: we must trust other measures, social measures, which of course are very disturbing.”
Risky but necessary bet
According to Nicola Stonehouse, professor of molecular virology at the University of Leeds, the strategy of not waiting at every step before starting production is a financially risky “gamble”.
But a necessary bet “in the current situation”, assures AFP.
The vaccine that the Oxford researchers are developing is based on a modified adenovirus that affects chimpanzees. “It generates a strong immune response with a single dose and it is not a replicating virus”, therefore “it cannot cause a continuous infection in the vaccinated individual”. This makes it “safer for children, the elderly” and patients with underlying diseases like diabetes, the researchers explain.
Widely criticized for its management of the health crisis, the Boris Johnson government created a task force last week to coordinate the researchers’ efforts and to be able to mass-produce a vaccine as soon as it becomes available, wherever it comes from.
It is also financially supporting research at Imperial College London, which hopes to start clinical trials in June.. His project focuses on a vaccine based on a different principle.
Finding a vaccine is the only possible way to return the world to “normality,” UN Secretary General Antonio Guterres warned last week, calling for an acceleration of projects under development.
On Monday, the UN adopted a resolution calling for “equitable, effective and rapid access” to a possible vaccine.
[ad_2]