
[ad_1]
If there is something that has revealed the crisis for Covid-19, it is the shortage of supply of medical devices and medical devices, not only due to high demand, but also due to the globalization of production to the detriment of local manufacturing. This is what has happened, among others, with invasive ventilators, essential to care for patients with bilateral pneumonia – a serious condition that causes the virus and causes respiratory failure and damage to other organs – in intensive care units (ICUs). ).
Given the situation, and coinciding with the peak of the epidemic and the saturation of hospitals, there was an “explosion of innovation” in March from universities, companies, hospitals and civil society to respond to this emergency. This is how AIRE (Innovative Aid to Breathing) emerged, a platform that launched on 12th of last month the challenge of manufacturing low cost and code-free, patent-free equipment that could be used in ICUs, says Jorge Barrero, one of its promoters and director of the Cotec Foundation.
Dependence on globalized supply chains, the focus of the problem
Hence the well-known prototype OxyGen was born, designed by the startup Protofy, manufactured by Seat and being tested at the Hospital Clínic de Barcelona and the Germans Trias i Pujol University. Or that of the Regional University of Malaga, which Fujitsu produces and which is being tested in 20 centers in Andalusia and 12 in Madrid. In total, according to Barrero, there are seven devices authorized by the Spanish Drug Agency (Aemps) to be tested on patients. There are five or seven others preparing the documentation required by the regulator and 50 in the initial phase, but he believes that they will not be developed because the bottleneck has passed.
“We are not promoters of any one in particular. We have facilitated the playing field, working with the Medicines Agency in defining the protocol for clinical trials and with the component supplier companies, one of the critical points, the globalization of supply chains has demonstrated its fragility ”, he indicates Barrero. The Ministry of Health confirms that there are currently five prototypes approved for clinical research in hospitals in Barcelona, Madrid and Andalusia.
The annual volume of the market is only 500 units
Another of them is an initiative jointly developed by Celera, the Rey Juan Carlos University (URJC) and Omron electronics as a technological partner, at the request of the materials engineer graduated from URJC Javier González, also linked to this network of young talent. “In a first stage it will be tested on 14 patients at the Hospital Universitario de Alcorcón. We have already manufactured 10 and, depending on demand, because the needs of the ICU have declined, fortunately, it is planned to extend it to Hospital 12 de Octubre, the University of Fuenlabrada and the Vall d’Hebron ”, explains Joaquín Rams, professor at the college. This project was also joined by Airbus to manufacture flowmeters, a component that is scarce and that measures the amount of air that enters the lung; and the Mapfre Foundation and the Community of Madrid for their financial support.
Peculiarity of the business
“Both companies are significantly increasing their production due to the pandemic and are doubling shifts, they operate 24 hours a day, 7 days a week. They are producing much higher production than usual. In some cases, if they have had difficulty finding suppliers of components for this production, they have received the support of the Ministry of Industry, “report from the Spanish Federation of Health Technology Companies (Fenin).
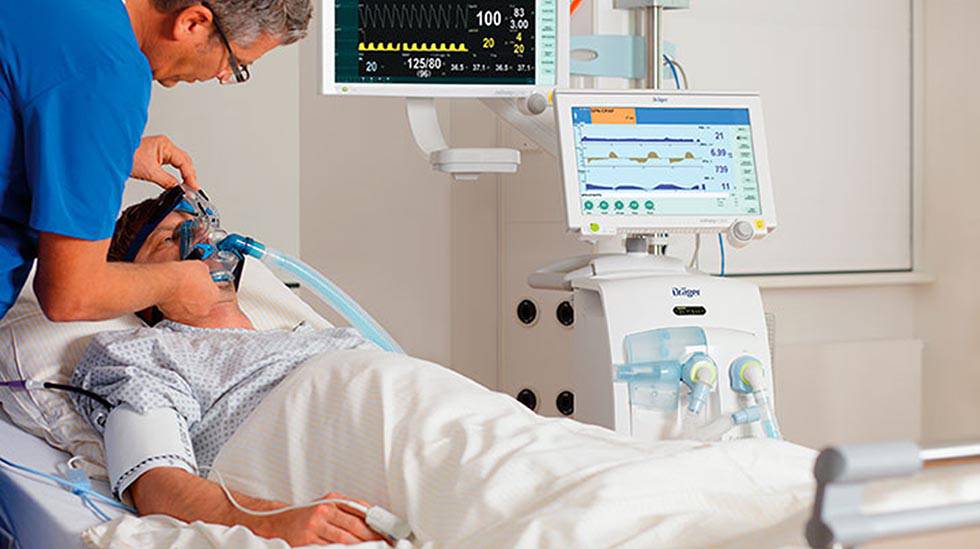
The multinationals Dräger, Medtronic, General Electric Healthcare, Getinge, Philips and Mindray also provide these technologies and provide maintenance and training, they add to the employer’s association. “We have always considered it important to strengthen the national industry, which has been relocated in the past for various reasons and which would contribute to improving the industrial fabric of our country with companies with a highly innovative profile, such as health technology, and generating highly qualified employment,” they say. .
Dräger, Medtronic, General Electric Healthcare and Philips, among the multinational providers
The most requested now is that of critics, which costs between 16,000 and 35,000 euros depending on its functionality and benefits, he says. “The demand for this type of equipment continues to be much higher today than the manufacturing capacity of the entire global industry, including accessories: hoses, filters, ventilation masks for emergency respirators that oxygenate the patient and are disposable”, points out. Therefore, he suggests adapting the use of other existing equipment, such as anesthesia machines or emergency respirators from the emergency services.
In Europe, the United States and Asia are the main manufacturers. The top 10 brands come from Germany, Switzerland, Sweden, Ireland, the USA and China. Thus, the world market moves around 100,000 respirators, excluding Asia, “there are a significant number of local manufacturers that only operate there.”
Relevant facts and curiosities

Purchases. To date, the Ministry of Health reports the acquisition of 293 invasive mechanical ventilation devices and 1,610 non-invasive units.
Manufacturers. Since April 3, the Hersill company, based in Móstoles (Madrid), started manufacturing 100 daily respirators. Health has signed the purchase of 5,000 equipment in the next two months, they add.
Certification The ventilators are very precise and complex invasive equipment, designed for use in life support of critically ill patients and so that they do not compromise the clinical state or the safety of patients and professionals, explain Fenin from the employers. “In the EU, these devices must have the CE marking, a mark that declares the conformity of the product with the safety, efficacy and quality requirements established in the legislation. In fact, their design, manufacturing procedure, functional tests, analysis of risks, clinical evaluation, conditioning material and information accompanying the product, among others, is evaluated by a notified body, “they detail.
Prototypes For the Spanish Medicines Agency to authorize these projects, they must first validate their effectiveness in an artificial lung and in animals (healthy and sick), taking into account the medical needs and characteristics of the disease, explains Joaquín Rams, professor at the King Juan Carlos. “They are rudimentary equipment, minimum technologies to be used during the emergency. If a commercial machine with CE marking has 3,000 pieces, they have 80 ”, says Jorge Barrero, from the Cotec Foundation.
Destinations. If not used in Spain, these low cost fans will be exported to Africa (Morocco) and South America.