News in Development
We are gathering more background on this news, stay tuned for updates.
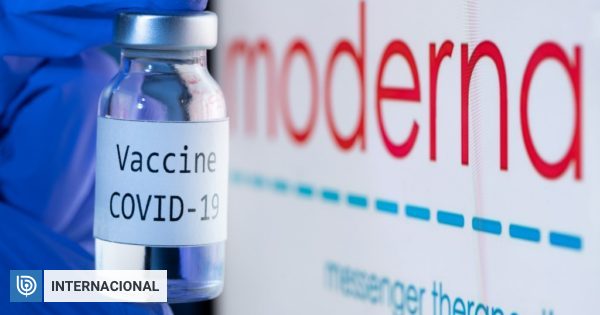
A panel of experts from the United States recommended this Thursday the emergency authorization of the Moderna’s covid-19 vaccine, paving the way for six million doses to begin shipping this weekend.
It is now expected that the Food and Drug Administration (FDA) imminently grant an emergency use authorization (USA), which would make this vaccine the second to be approved in a Western country, after the one developed by Pfizer / BioNTech.
This is good news in the face of the mass vaccination process that begins progressively in several countries, with the aim of stopping the contagion of coronavirus, which has caused millions of deaths in more than a year.
The Chilean ISP authorized this Wednesday the emergency use of the Pfizer / BioNTech vaccine, with which it is expected that in the next few days 20,000 doses destined for health personnel will land.