[ad_1]
The Institute of Public Health notified the voluntary withdrawal of two new contraceptives of the market, as well as the temporary suspension of registrations by Laboratorio Andrómaco SA, which produces and distributes the drugs.
This after detecting a quality problem in contraceptives Minigest-15 and Minigest-20, related to a lower amount of the active substance than declared, observed in some batches that have been distributed in the country.
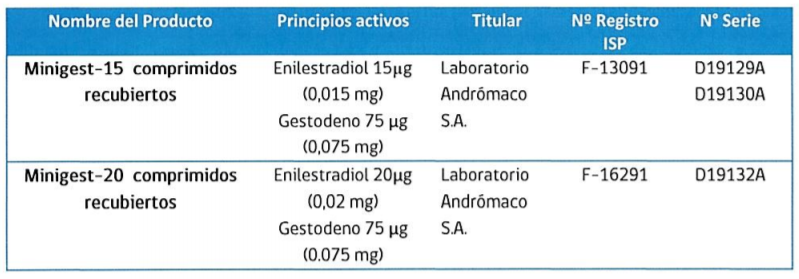
According to the ISP statement, the decrease in potency was identified when stability studies were carried out for both contraceptives, for which reason there are currently other pharmaceutical products registered with these active ingredients and in the doses mentioned, which can be verified in the institute’s website
In this way, recommendations were given to those who consumed the products, indicating that they contact their midwife, doctor or pharmacist to rreceive indications regarding the continuity and alternatives of oral contraceptives available.
Additionally, you are urged to use additional, non-hormonal protective measures to prevent a possible unplanned pregnancy, until you can have access to an oral contraceptive to replace the one you were using.
The letter also stipulates that it is possible that during the change of oral contraceptiveshow irregularities in your menstrual cycle, which corresponds to a frequently observed reaction when starting a new treatment, and usually improves with the passage of time.
[ad_2]