Bay Area residents are joining the global race for a coronavirus vaccine this week, with developers of two of the most promising candidates seeking hundreds of volunteers in San Francisco, Oakland and Santa Clara County.
Kaiser Permanente enrolled its first participants in Northern California in a worldwide clinical trial for a vaccine made by Pfizer and the German technology company BioNTech on Monday. In San Francisco, researchers with UCSF and the Department of Public Health expect new volunteers to start vaccinating through AstraZeneca and Oxford University next week.
Sutter Health’s East Bay AIDS Center, located at Alta Bates Summit Medical Center in Oakland, is also expected to participate in the AstraZeneca trial.
Both experimental vaccines were included in the Phase 3 studies, the final step for federal approval, assuming the products prove safe and effective. In all, about 1,400 Bay Area participants are expected to enroll in both trials, but given the speed at which vaccine development is happening, scientists expect many more hundreds, if not thousands, of volunteers in the coming months.
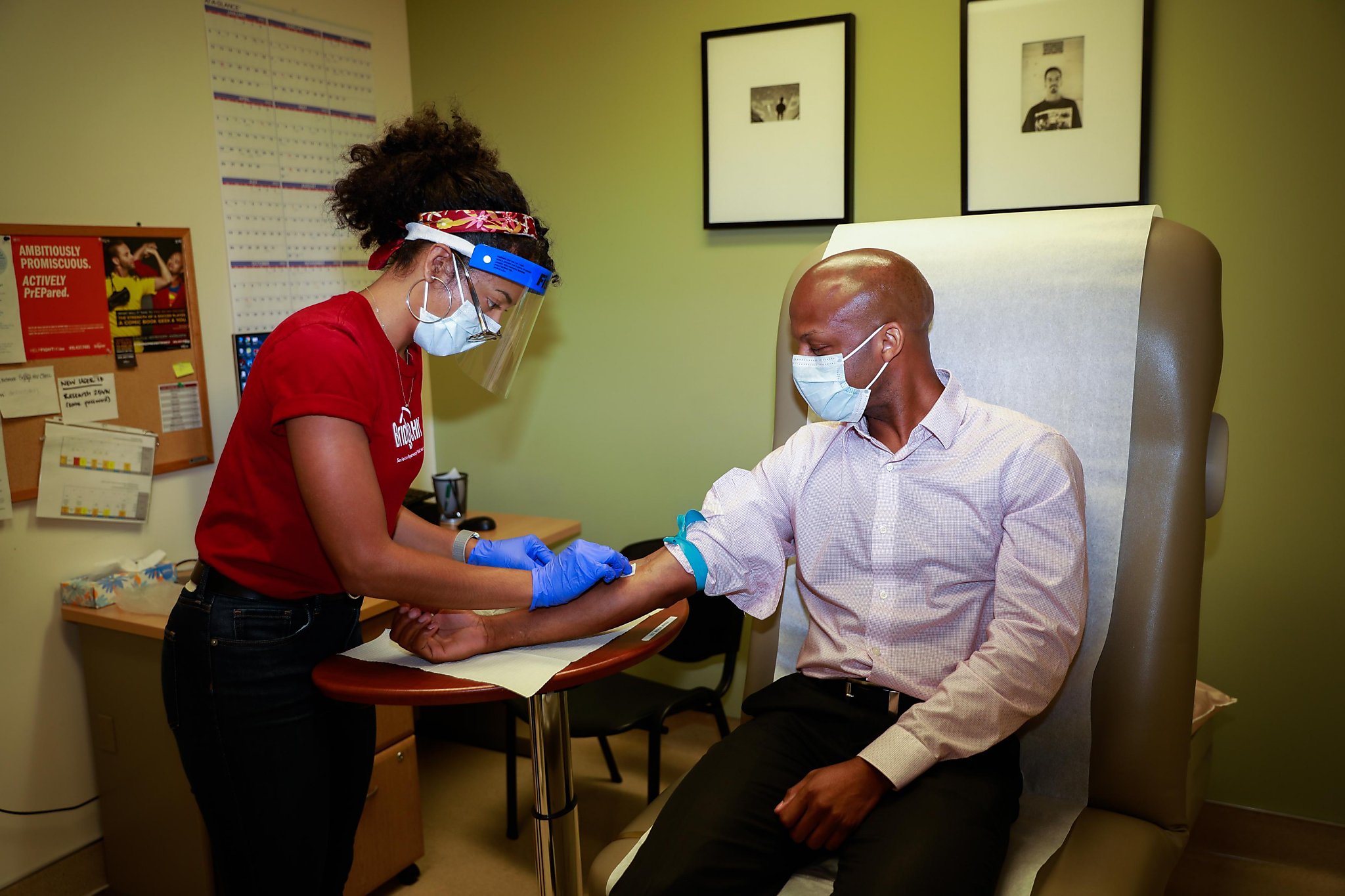
“This pandemic has had an enormous negative impact on every aspect of society and our daily lives. It caused so much pain and suffering. Being part of the solution is very exciting, “said Dr. Susan Buchbinder, co-lead researcher of the AstraZeneca vaccine tests in Bay Area and director of Bridge HIV, the San Francisco public health HIV prevention unit.
“Although we will be testing AstraZeneca, we expect to be part of other trials as well,” she said. “I would be happy if one of the faxes is successful. I have root for them all. ”
Global vaccine development has accelerated since the new coronavirus was identified in early January. More than 100 products are being tested, and dozens have been tested in humans.
The coronavirus has spread around the world, infecting more than 20 million people, including 5 million in the US, and killing 745,000 worldwide. Many experts on infection believe that vaccines are the best, and perhaps only, hope for ending the pandemic and revamping economies shaken by social constraints without further devastating loss of life.
The United States has identified five vaccine candidates that they consider to be the most viable. In addition to the faxes Pfizer and AstraZeneca, the US bets on products made by Johnson & Johnson, Merck and Moderna. The faxes Pfizer, AstraZeneca and Moderna are all in Phase 3 studies, and collectively plan to register nearly 100,000 volunteers worldwide.
The U.S. Department of Health and Human Services has already allocated up to $ 1.95 billion to Pfizer to begin making 100 million doses of vaccine and up to $ 1.2 billion to AstraZeneca for 300 million doses. Producing vaccines while they are still being tested is risky, but the hope is that they can be given away immediately to the public if they prove safe and effective.
“These are heroic efforts, truly noble efforts to combat this pandemic,” said Dr. Bali Pulendran, a vaccine specialist at Stanford Health Care. “And I find it wonderful that these premier Bay Area institutions are playing a very active role in this effort.”

Both the AstraZeneca and Pfizer studies are double-blind and placebo-regulated, meaning that some participants will receive the vaccine and others a placebo. All participants will receive two doses by injection, one month apart. Neither participants nor researchers will know who received the vaccine.
The trials will last two years, but researchers expect to have results a few months early. The AstraZeneca trial is part of the National Institute for Health COVID Prevention Network. People can register online to participate in coronaviruspreventionnetwork.org. The Pfizer trial is sponsored by the fax developers. Only Kaiser members can initially participate in the study in California.
The aim of both trials is to determine whether the vaccines prevent humans from getting COVID-19, the disease caused by the new coronavirus. The studies will also look at the long-term safety of the vaccines, and whether the vaccines trigger a strong immune response and prevent severe infection. Early studies of both vaccines found that they were safe, leaving only mild short-term symptoms such as fatigue or body aches in some volunteers.
The fax tests brought to the Bay Area apply to existing research networks that have been used for decades to research immunization for HIV and a variety of other infectious diseases.
“In some ways (the trial) it is very similar to the type of vaccine tests we do all the time. What is really not typical is the speed at which all this took place, ”said Dr. Nicola Klein, director of the Kaiser Permanent Vaccine Study Center and lead researcher for the trial in Northern California, who enrolls participants in Santa Clara County and Sacramento.
It is also not known how long immunity can last, assuming the vaccines work. The Food and Drug Administration has said that each vaccine must be at least 50% effective to be considered for approval.
Dusta Eisenman, a daycare worker at Kaiser Santa Clara, received her first dose in the Pfizer study on Wednesday morning. She said that although she’s a little nervous’ about being one of the first participants in the trial, ‘I push that down for the bigger good.’
‘I understand it’s warp speed and it’s going really fast, but we have a lot of bad people dedicated to it. I put my trust in it, ‘she said. ‘To feel that I’m contributing something now, and maybe there’s a light at the end of the tunnel, that’s exciting for me. I try to be a light to friends and others who feel hopeless at this point. ”
Erin Allday is a staff writer for the San Francisco Chronicle. Email: [email protected] Twitter: @erinallday