[ad_1]
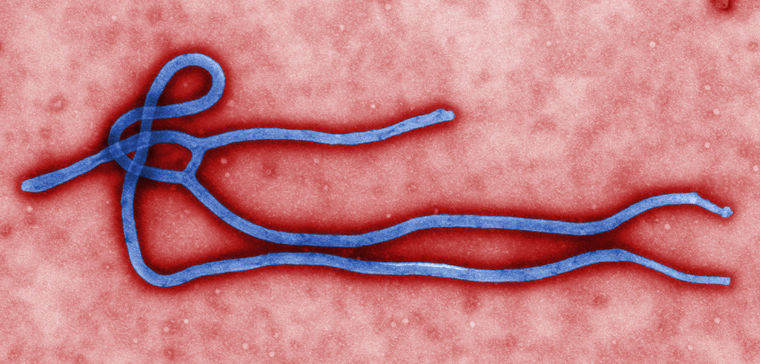
A virus that normally infects animals makes the leap to humans, whose immune systems have never seen it before. Suddenly it spreads across the world, leaving death and chaos in its wake. We are living with that reality now and have previously gone through it with HIV, SARS, MERS, Ebola, Hanta and various flu viruses that have threatened humanity in recent decades.
While there are many organizations trying to stay on top of emerging disease threats, it would be helpful if we could identify the main sources of potential threats. If, for example, we knew that certain species were more likely to carry viruses that could make the leap to humans, we could examine the viruses found in those species, identify the main threats, and even develop therapies or vaccines in advance.
But a study recently published in PNAS suggests that there is no real pattern of where humans are detecting new viruses. By contrast, groups with many species tend to have many viral species, and those make the leap to humans largely in proportion to the number of species.
Zoonotic risk
A disease that can be transmitted from animals to people is technically called “zoonotic.” Although there are a variety of diseases that incorporate time in other species as part of their lifestyle (malaria is a classic example), the risk that concerns us is a virus that normally circulates within a non-human species but develops the ability to spread within humans and leave its original host behind.
These types of events are relatively common. Flu viruses seem to jump between us and our agricultural species with some regularity. Other viruses, such as members of the hantavirus family, appear to frequently make the jump to humans without establishing the ability to spread from human to human.
It is the latter characteristic that creates the risk of a global pandemic. Two previous coronaviruses, SARS-CoV-1 and MERS, did not spread as widely as humans as SARS-CoV-2, allowing containment methods to stop their spread before a pandemic could develop.
Are there species that could be especially good launching pads for a pandemic? A couple of hypotheses suggest that this might be the case. One hypothesis is that evolutionary distance is important. A virus that normally circulates in a human-related species is more likely to have components that can interact more effectively with proteins present in human cells. If this were the case, we would probably expect to see more zoonotic leaps of viruses infecting our fellow primates.
An alternative idea has emerged from the fact that this does not seem to be always true. Bats, for example, have humans “endowed” with viruses as distant as SARS-CoV-1 and Ebola, and they are not especially related to us. As a result, researchers have hypothesized that there could be what have been called “special reservoirs,” or species that, for ecological or lifestyle reasons, have ended up with viruses that can more easily adapt to human hosts. These special deposits may be more likely to live close to humans, increasing the risk of transmission.
Two Glasgow researchers, Nardus Mollentze and Daniel Streicker, decided to test these two hypotheses by determining whether any group of species was more likely to spread viruses to humans.
Building trees
To do so, Mollentze and Streicker built a comprehensive database of each virus that reportedly made the jump to humans, as well as the host it jumped from. In total, there were 415 different viruses that were assigned a host and could be used for analysis (ie, 673 known virus species). These were distributed into 30 families (the designation two levels above the species) and had come from 11 different orders of host species (one order is the level above the family).
On their own, the results would seem to point to the special reservoir model, as hoofed ungulates (like our farm animals) and rodents collectively accounted for half of the viruses that had become human hosts. But things became more complex when the authors attempted to analyze the properties of a virus that made it more likely to make this transition. The best combination of properties, which could explain about half the probability of a zoonotic jump, was dominated by things like transmission through insects and a relatively simple replication cycle within cells.
A time when the order of the host in the evolutionary tree seemed to matter at first, much less so once the authors adjusted for a critical factor: how many individual species make up that order. For example, rodent and ungulate species can transmit more viruses to us, but there is a lot of species in these groups. If you adjust the rate by species number, the effect largely disappears. If you also control for the fact that we have identified many more species of viruses in mammals than in birds, then the effect becomes little more than statistical noise. The probability that a group of species transmits a virus to humans becomes a function of how many species are in that group.
This is inconsistent with the special reservoir hypothesis. But things don’t look very good for evolutionary explanation either. Although the zoonotic risk decreased as you moved away from the primates, this represented less than 1 percent of the overall risk.
In fact, if you simply calculated the number of zoonotic jumps based on the number of species, the groups that seemed threatening begin to seem quite mundane. Rodents, for example, are expected to have given 42 viruses to humans; We know of 41 cases in which this occurred. We would expect the bats to have transferred 28 viruses to us, but they have only sent 22 to us. The only exception is, once again, the ungulates, which seem to send viruses at a higher rate than expected.
Now what?
The hope was that by identifying the rules for zoonotic transfers, we would be able to identify groups of species that are at high risk of causing problems and could therefore be more carefully monitored. This analysis suggests that these groups may not exist. It does not rule out the possibility that there are groups of species below the order level that are critical points for zoonotic transfers. But at this point, the number of viruses transferred per group is likely to be small and not stand out from the statistical noise.
That said, some species / virus combinations are remarkable. For example, while bats are notable for having been the source of SARS-CoV-1 and Ebola, they are actually more likely to transfer a new species of rabies virus to humans. Other primates are an important source of adenovirus and dengue species, while rodents tend to transfer hantaviruses and arenaviruses.
While this is not especially good news for specific surveillance efforts, it might not generally be bad news. Having obvious goals could mean that we focus too much on them, leaving us vulnerable to risks we hadn’t anticipated.
PNAS, 2020. DOI: 10.1073 / pnas.1919176117 (About DOIs).