[ad_1]

Photo illustrated by Reuters
- A key part of the regulatory approval process for the Johnson & Johnson Covid-19 vaccine got under way on Friday.
- The company has been sharing data with South African regulators for months.
- There is no evidence that a similar requisite request has been made for the Oxford / AstraZeneca vaccine, even though doses are expected to arrive within days.
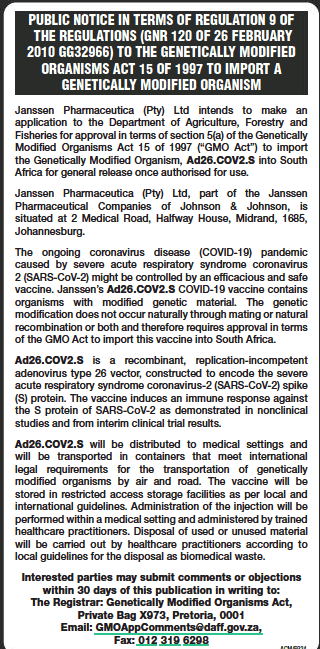
A key part of the regulatory approval process, an application for a genetically modified organisms (GMO) permit, for Johnson and Johnson’s Covid-19 vaccine went live on Friday, making it the first confirmed vaccine to undergo this process. .
Meanwhile, the health department has refused to clarify the status of a similar request for the Oxford / AstraZeneca vaccine, which is expected to arrive in a few days from the Serum Institute of India (SII).
News24 previously reported that the request for a GMO permit for the IBS vaccine had not yet been submitted as of January 15, casting doubt on Health Minister Zweli Mkhize’s schedule for the arrival and distribution of the first injections of Covid-19 vaccine in the country.
He reiterated this week that the first million doses of the Oxford / AstraZeneca vaccine would arrive before the end of the month. However, the lack of clarity surrounding the GMO permit application has led to uncertainty as to whether, upon arrival, the doses may need to be stored in a warehouse for at least 30 days prior to distribution.
It is not clear if this 30-day period will not apply to doses of vaccine made for IBS. The health department was first asked to clarify this issue on January 14, but beyond denying any oversight on its part, it did not respond to inquiries sent Tuesday.
A search of national publications has not revealed any announcement of a notice like the one published about the J&J vaccine.
An industry expert told News24 that customs may not clear the shipment without the necessary GMO permission. It is understood that the Pfizer / BioNTech and Moderna Covid-19 vaccines would not require a GMO permit due to the difference in the types of vaccines they produce.
READ | The 4 types of Covid-19 vaccine technologies and how they work
On Friday, Mkhize announced that the South African Health Products Regulator (SAHPRA) had given approval to the health department to purchase IBS vaccines.
He did not speak about the GMO permit issue or its enforcement, which News24 understands the Biovac Institute had a mandate to secure.
30-day public participation period
National newspapers on Friday and Monday carried a notice published by the GMO Registrar, inviting the public to provide comments and input on the approval of a permit for the importation of J&J vaccine, a requirement of the GMO Act.
The GMO registrar is located within the Department of Agriculture, Agrarian Reform and Rural Development (DALRRD).
By law, the public participation period is 30 days, after which a committee reviews contributions and comments, as well as technical information before making a decision.
The GMO permit is required before importing or “general use” of any GMO, whether medicinal or agricultural.
In October, a similar notice was published for the J&J vaccine, for a permit allowing the import of doses for phase three trials of the vaccine at the local level. South Africa participated in a global phase three trial with 45,000 participants.
It is not clear whether a permit was obtained for the Oxford / AstraZeneca vaccine trials conducted in the country, which began in June 2020. Online searches of national newspapers did not reveal any published advisories.
According to an industry expert, GMO approval can take up to three months under normal circumstances, but accelerated approval processes in response to the Covid-19 pandemic can shorten this considerably.
SAHPRA may approve vaccines for emergency use under Section 21 of the Drug and Related Substances Act, but approval of Section 21 and approval of the GMO permit are codependent, as News24 understands from the explanations provided by SAHPRA and DALRRD.
J&J has yet to seek similar approvals for its vaccine in the US and elsewhere, but News24 understands that the company has been sharing data with South African regulators during the clinical trial phases, with a view to shortening delays around to approvals.
Results of the company’s phase three tests, which included participants from South Africa, are expected to be released this week or in the first week of February.
It is one of the most closely monitored vaccines as a result of early data indicating that it was effective with a single dose, compared to the two-dose regimens required with other vaccines.
In November, President Cyril Ramaphosa announced that Aspen Pharmacare would produce 300 million doses of the J&J vaccine at its Port Elizabeth factory.
Health officials earlier this month confirmed a nine million dose deal with J&J, but the regulatory process that began this week could see regulators greenlight the vaccine only in late February.
Do you want to know more about this topic? Sign up to receive one of 33 News24 newsletters to receive the information you want in your inbox. Special newsletters are available to subscribers.