[ad_1]
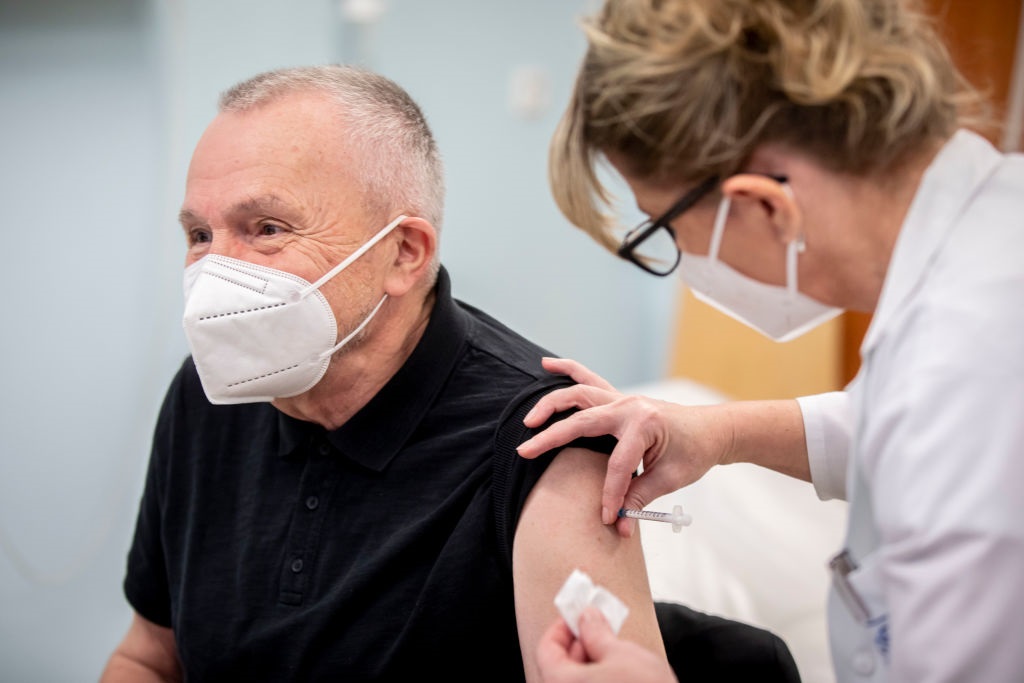
- A vaccine developed by the US company Pfizer and the German company BioNTech was approved for use in Britain on December 2.
- A total of 16 countries and the European Union have given the green light to the vaccine.
- AFP discusses the availability, efficacy, and side effects of jabs.
European countries are following the United States, Britain, and a handful of other countries to start rolling out coronavirus vaccines.
The rapid development and approval of the drugs has been acclaimed around the world, doubts remain about the availability, efficacy, and side effects of jabs.
READ | Covax: Who will be the first in South Africa to get the vaccine?
Typically, it takes about 10 years to develop and commercialize a new vaccine, but the process was greatly sped up for Covid-19.
A vaccine developed by the American company Pfizer and the German company BioNTech was approved for use in Britain on December 2.
Since then, thousands of older people have received the first dose.
A total of 16 countries and the European Union have given the green light to the Pfizer-BioNTech vaccine.
The US Food and Drug Administration granted emergency authorizations to the drug Pfizer-BioNTech and another coup from the American company Moderna.
Russia began vaccinations on December 5 with its national drug Sputnik V, which is still in its third phase of clinical trials.
China has already given the green light for emergency use of some of its vaccines, although none have been formally approved.
A total of 16 vaccines are in the final stage of development, including those already on the market, says the World Health Organization.
Vaccines can start from the Sunday following approval of the Pfizer-BioNTech jab by the European Medicines Agency (EMA).
Each member country will take the lead in defining its priorities with the launch.
But three member states – Germany, Hungary and Slovakia – started vaccinations one day early on Saturday.
Since November 9, four manufacturers have announced that their vaccine is effective: Pfizer-BioNTech, Moderna, the British alliance AstraZeneca-University of Oxford and the Russian state institute Gamaleia.
These announcements are based on phase 3 clinical trials involving tens of thousands of volunteers.
However, detailed and validated data is available only for Pfizer-BioNTech and AstraZeneca-Oxford drugs.
The scientific journal The Lancet confirmed on December 8 that the AstraZeneca vaccine was 70% effective on average.
The FDA confirmed that the Pfizer-BioNTech vaccine is 95% effective and Moderna claims that its drug is 94.1%. Russia claims 91.4% efficacy for its Sputnik V vaccine.
The AstraZeneca-Oxford vaccine is the least expensive and costs around EUR2.50 per dose. Moderna and Pfizer / BioNTech vaccines have a logistical drawback, as they can only be stored long-term at very low temperatures (-20 degrees Celsius for the former, -70 degrees Celsius for the latter).
Experts insist that clinical trials carried out on tens of thousands of volunteers would have already detected any significant risks. But rarer side effects or those affecting specific patient profiles cannot be ruled out.
According to the FDA, the Pfizer-BioNTech vaccine can cause painful reactions in the arm where the injection is given. Other undesirable side effects include fatigue, headaches, cramps and, more rarely, fever.
The most important is long-term effectiveness.
Penny Ward from King’s College London said the key questions were how long would protection last and could the virus eventually mutate and no longer be covered by the vaccine?
Another crucial question is whether vaccines act differently in higher-risk populations, starting with older people who are most likely to develop a severe form of Covid-19.
It also remains to be seen whether these vaccines block the transmission of the virus and also reduce the severity of the disease in those who have received the vaccine.
European Union experts believe that current Covid-19 vaccines remain effective against the new strain of the virus detected in Britain and elsewhere, which is believed to be more infectious.
“At the moment there is no evidence to suggest” that the Pfizer-BioNTech vaccine “is not effective against the new variant,” the European Medicines Agency said.
The co-director of the German laboratory BioNTech, Ugur Sahin, echoed that message, adding that in any case, his company would be in a position to provide a vaccine for a new strain of Covid-19 in six weeks.