
The built-in solar flow battery device contains a solar cell to convert sunlight into electricity and chemicals that can store electricity for later use. Credit: Wenjie Li
Chemists at the University of Wisconsin-Madison and their collaborators have created a highly efficient and durable solar flux battery, a way to generate, store and forward renewable electricity from the sun in one device.
The new device is made of silicon solar cells combined with advanced solar materials integrated with optimally designed chemical components. The solar flow battery, manufactured by the Song Jin Laboratory in the UW-Madison chemistry department, achieved a new record efficiency of 20 percent. That beats most commercially available silicon solar cells in use today and is 40 percent more efficient than the previous record holder for solar flow batteries, also developed by the Jin Lab.

Schematic illustration of an integrated solar flow battery. A solar cell (in green) is connected to chemical tanks (in red and blue) that can store electricity for later use. Credit: Wenjie Li
While solar flux batteries are years away from commercialization, they offer the potential to provide reliable electricity storage and generation for lighting, cell phones, or other critical uses for homes in remote areas. They combine the advantages of photovoltaic cells that convert sunlight into electricity with the advantages of flow batteries, which use tanks of chemicals that can react to produce electricity and be recharged by solar cells.
The researchers published their work today (July 13, 2020) in the journal Natural materials. UW-Madison graduate student Wenjie Li is the study’s lead author. The Jin Laboratory collaborated with researchers from the University of New South Wales and the University of Sydney in Australia, Utah State University, King Abdullah University of Science and Technology in Saudi Arabia, and Hong Kong City University .

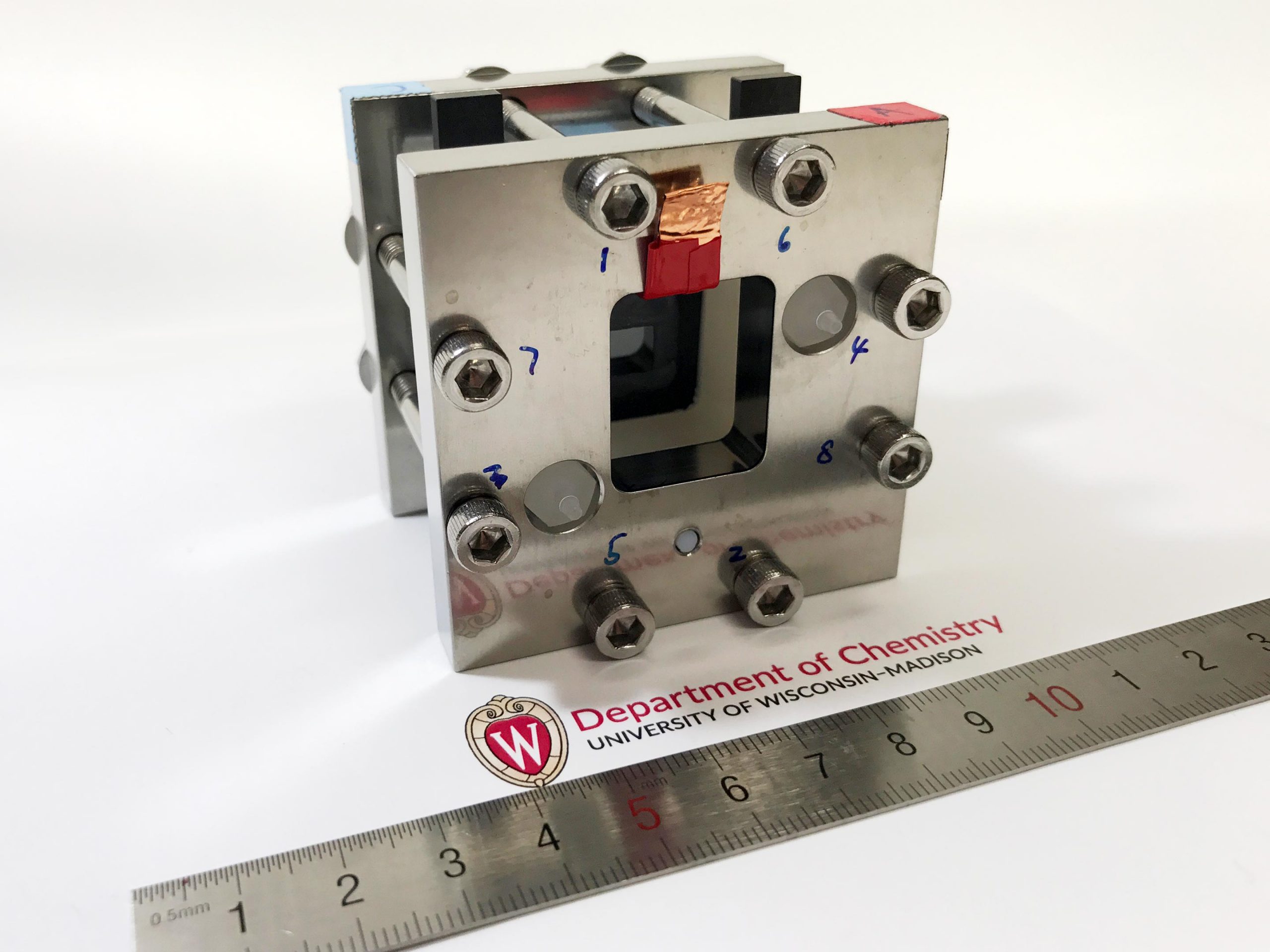
Experimental solar device in the laboratory of the University of Wisconsin-Madison. Credit: Wenjie Li
Since the sun doesn’t always shine, storage is key to practical solar electricity, especially in remote, rural regions with lots of sunlight, such as in the sunbelt regions of the US, Australia, Saudi Arabia, and Africa. Many home solar systems use leadacid or lithium ion batteries for storing electricity. Flow batteries, which use large tanks of liquid chemicals to store energy, may be less expensive on a larger scale and are an ideal storage option to merge with solar cells.
Jin Laboratory has spent years studying and improving integrated solar flow battery systems. In 2018, it developed a solar flux battery using a triple layer of efficient but expensive solar materials that achieved an overall efficiency of 14 percent. However, corrosion greatly reduced the life of the device.
In their latest report, the researchers turned to an increasingly popular material for photovoltaic cells, halide perovskites. The solar conversion efficiency of these specialty materials has increased dramatically from a small percentage to over 25 percent in 10 years. Recent research has shown that halide perovskites can also increase the efficiency of traditional silicon solar cells by capturing more energy from the sun.
This new generation of highly efficient silicon perovskite solar cells is on the way to commercialization. However, silicon is still key to making a stable device that can withstand chemicals in a flow battery.
“Our motivation for designing the solar cell was to combine these two materials so that we have high efficiency and good stability,” says Li.

Perovskite structure. Credit: Wenjie Li
Professor Anita Ho-Baillie and postdoctoral researcher Jianghui Zheng in Australia made perovskite-silicon solar cells with an additional layer of protection on the silicon surface. They sent the solar cells to Wisconsin for testing.
To predict the ideal voltage at which flow batteries should operate, Li developed a new theoretical modeling method. Modeling allowed him to select a couple of chemicals in the flux battery that would operate at the ideal voltage based on the characteristics of the solar cell, maximizing efficiency. The chemicals are organic compounds, not expensive metals like in traditional flow batteries, and they dissolve in a benign solution of table salt water instead of strong acids.
The chemistry professor at Utah State University, T. Leo Liu, and his graduate students provided the key chemicals. Thanks to a good match between the solar cell and the flow battery, the winning device maintained high efficiency for hundreds of hours and hundreds of charge and discharge cycles, while retaining most of its capacity. That lifespan was several times longer than previous devices. Overall, the new system’s long life span and 20 percent efficiency made it the best solar flow battery device to date.
“That’s 20 percent efficiency whenever you want it,” says Jin. “You can use solar electricity right away during the day and get 20 percent, or you can use it at night from storage and get 20 percent.”
There is still a lot of research to be done before such devices make practical renewable energy solutions. Increasing the size and scale of today’s small devices in the research lab is challenging. And even though the researchers created a relatively durable battery, real-world applications demand even greater robustness. Jin Lab continues to develop even more efficient solar flux batteries while experimenting with practical solutions to reduce the cost of the devices.
That research could one day produce a new way to harvest, store and use energy from the sun.
“Our ultimate goal, if we can make it practical, is to target home solar systems,” says Li. “People who don’t have access to the electrical grid could use this device for reliable electricity.”
###
Reference: July 13, 2020, Natural materials.
DOI: 10.1038 / s41563-020-0720-x
This work was supported in part by the National Science Foundation (grant 1847674).