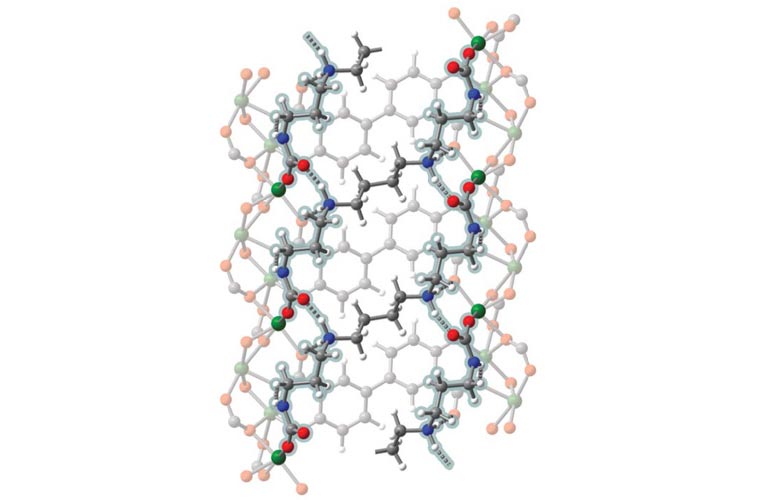

The metal-organic frames are highly porous, making them ideal for absorbing gases and liquids. This graph shows the interior of a magnesium metal-based MOF (green balls), and has added molecules – tetraamines (blue and gray) – added to the pores to more efficiently absorb carbon dioxide from plant emissions of energy. Credit: UC Berkeley, graphic by Eugene Kim
Modified tetraamine MOF eliminate 90% of CO2 more efficiently and economically.
A breakthrough in carbon capture technology could provide an efficient and economical way for natural gas power plants to remove carbon dioxide from their smoke emissions, a necessary step to reduce greenhouse gas emissions for curb global warming and climate change.
Developed by researchers from the University of California, Berkeley, Lawrence Berkeley National Laboratory and ExxonMobil, the new technique uses a highly porous material called a metal-organic framework, or MOF, modified with nitrogen-containing amine molecules to capture COtwo and steam at low temperature to remove COtwo for other uses or to kidnap it underground.
In experiments, the technique showed six times greater ability to remove COtwo of flue gas than current amine-based technology, and was highly selective, capturing more than 90% of COtwo Emitted The process uses low temperature steam to regenerate the MOF for repeated use, which means less energy is required for carbon capture.
“For the COtwo steam capture, extraction: where you use direct contact with steam to remove COtwo – It has been a kind of holy grail for the field. It’s rightly viewed as the cheapest way to do it, “said lead researcher Jeffrey Long, professor of chemistry and of chemical and biomolecular engineering at the University of Berkeley at Berkeley Lab.” These materials, at least from the experiments we’ve conducted so far, they look very promising. “
Because there is little market for most of the captured COtwo, the power plants would probably pump most of it to the ground, or hijack it, where it would ideally become rock. The cost of eliminating emissions would have to be facilitated by government policies, such as carbon trading or a carbon tax, to incentivize COtwo capture and kidnapping, something that many countries have already implemented.
The work was funded by ExxonMobil, which is working with both the Berkeley group and Long’s new company, Mosaic Materials Inc., to develop, scale and test processes to remove COtwo of emissions.
Long is the lead author of an article describing the new technique that was published in the July 24, 2020 issue of the magazine. Science.

The single-pore atomic structure in a MOF showing how carbon dioxide molecules (gray and red spheres) bind to tetraamines (blue and white spheres), building a CO2 polymer that passes through the pore. Low-temperature steam can remove carbon dioxide for sequestration, allowing the MOF to be reused to capture more carbon from emissions from the power plant. Credit: UC Berkeley, graphic by Eugene Kim
“We were able to make the initial discovery, and through research and testing, we derived a material that in laboratory experiments has shown the potential not only to capture COtwo under the extreme conditions present in flue gas emissions from natural gas power plants, but to do so without loss of selectivity, “said co-author Simon Weston, principal research associate and project leader at ExxonMobil Research and Engineering Co.” demonstrated that these new materials can be regenerated with low-grade steam for repeated use, providing an avenue for a viable solution for carbon sequestration at scale. “
Carbon dioxide emissions from fossil fuel burning vehicles, power plants and industry account for approximately 65% of the greenhouse gases that drive climate change, which has already increased the average temperature of the Earth in 1.8 degrees. Fahrenheit (1 degree Celsius) since 19th century. Without a decrease in these emissions, climate scientists predict increasingly high temperatures, more erratic and violent storms, several feet of sea level rise, and the resulting droughts, floods, fires, famines, and conflicts.
“Actually, the kind of thing the Intergovernmental Panel on Climate Change says we must do to control global warming, COtwo Catching is a big part, “said Long. “We don’t have a use for most of the COtwo that we need to stop broadcasting, but we have to. “
Peel
Power plants pull COtwo of smoke emissions today by bubbling flue gases through organic amines in the water, which bind and remove carbon dioxide. The liquid is then heated to 120-150 C (250-300 F) to release the COtwo gas, after which the liquids are reused. The entire process consumes around 30% of the energy generated. Sequestering the captured COtwo the subfloor costs an additional, albeit small, fraction of that.

An electricity generating plant powered by natural gas. A new technique could capture carbon dioxide from the emissions of such plants to sequester it underground and reduce the greenhouse gases responsible for climate change. Credit: Courtesy of the International Energy Agency.
Six years ago, Long and his group at the UC Berkeley Gas Separation Center, funded by the United States Department of Energy, discovered a chemically modified MOF that easily captures COtwo of concentrated emissions of smoke from power plants, potentially reducing the cost of capture in half. They added diamine molecules to a magnesium-based MOF to catalyze the formation of CO polymer chainstwo which could then be purged with a wet stream of carbon dioxide.
Because MOFs are very porous, in this case like a honeycomb, a clip’s weight amount has an internal surface equal to that of a soccer field, all available to adsorb gases.
A major advantage of MOFs added to amine is that amines can be adjusted to capture COtwo at different concentrations, ranging from 12% to 15% typical of coal plant emissions to 4% typical of natural gas plants, or even much lower concentrations in ambient air. Mosaic Materials, which Long co-founded and directs, was created to make this technique widely available to industrial and power plants.
But the current of 180 C of water and COtwo necessary to remove captured COtwo eventually ejects the diamine molecules, shortening the life of the material. The new version uses four amine molecules, one tetraamine, which is much more stable at high temperatures and in the presence of steam.
“Tetraamines are so tightly bound within the MOF that we can use a very concentrated stream of water vapor with zero COtwo, and if you tried with the above adsorbents, the steam would start to destroy the material, “Long said.
They showed that direct contact with steam at 110-120 C, slightly above the boiling point of water, works well to remove COtwo. Steam at that temperature is readily available in natural gas power plants, while 180 C COtwo-the water mixture required to regenerate the previous modified MOF required heating, which wastes energy.
When Long, Weston, and their colleagues first thought about replacing diamines with harder tetraamines, it seemed like a long shot. But the crystal structures of diamine-containing MOFs suggested that there could be ways to connect two diamines to form a tetraamine while preserving the material’s ability to polymerize CO.two. When UC Berkeley graduate student Eugene Kim, the paper’s first author, chemically created the MOF with tetraamine, he outperformed the MOF with diamine on the first try.
The researchers then studied the structure of the modified MOF using Berkeley Lab’s advanced light source, revealing that COtwo The polymers that line the pores of the MOF are actually bound by tetraamines, like a ladder with tetraamines as stepping stones. Density functional theory calculations of first principles using the Cori supercomputer at the Berkeley National Center for Scientific Research in Energy Research (NERSC), computer resources at the Molecular Foundry and resources provided by the Computer program The Berkeley Research Campus confirmed this remarkable structure that Long’s team had initially envisioned. .
“I’ve been researching Cal for 23 years, and this is one of those moments where you have what seemed like a crazy idea, and it just worked right away,” Long said.
Reference: “Cooperative Carbon Capture and Vapor Regeneration with Organic Metal Structures with Tetraamine Addition” by Eugene J. Kim, Rebecca L. Siegelman, Henry ZH Jiang, Alexander C. Forse, Jung-Hoon Lee, Jeffrey D. Martell , Phillip J Milner, Joseph M. Falkowski, Jeffrey B. Neaton, Jeffrey A. Reimer, Simon C. Weston and Jeffrey R. Long, July 24, 2020, Science.
DOI: 10.1126 / science.abb3976
Co-authors with Long, Kim, and Weston are ExxonMobil’s Joseph Falkowski; Rebecca Siegelman, Henry Jiang, Alexander Forse, Jeffrey Martell, Phillip Milner, Jeffrey Reimer and Jeffrey Neaton of UC Berkeley; and Jung-Hoon Lee of Berkeley Lab. Neaton and Reimer are also senior faculty scientists at Berkeley Lab.